
ncert solutions, solutions of ncert, ncert solutions for, ncert solutions online, solutions ncert, online ncert solutions , organic chemistry – some basic principles and techniques 11 notes, class 11 chemistry notes, organic chemistry – some basic principles and techniques class 11, organic chemistry – some basic principles and techniques class 11 notes, class 11 organic chemistry – some basic principles and techniques, note chemistry, chemistry notes, organic chemistry – some basic principles and techniques, class 11 cchemistry chapter 12 notes, 11th standard chemistry notes, 11th std chemistry notes, class 11 chemistry notes chapter 12, organic chemistry – some basic principles and techniques chapter class 11 notes
Organic compounds are the hydrocarbons and their derivatives and organic chemistry is that branch of chemistry that deals with the study of these compounds
The atomic number of Carbon is 6 and its electronic configuration is 2,4 i.e. it has 4
valence electrons. Thus carbon is always tetracovalent, i.e. it forms 4 covalent
bonds with other atoms
Due to tetravalency of carbon it has a tetrahedron shape.
Catenation- The self linking property of carbon is known as catenation. This is the
main reason of existence of such large number of compounds
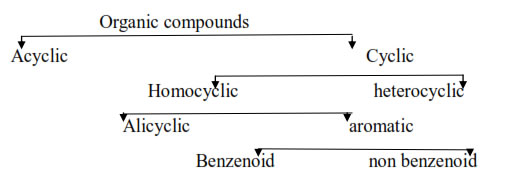
Classification of organic compounds
Functional groups:A functional group may be defined as an atom or a group of atoms present in a molecule which largely determines the chemical properties.
CLASS OF ORGANIC COMPOUNDS |
NAME OF FUNCTIONAL GROUP |
STRUCTURE |
Alkenes |
double bond |
=C=C= |
Alkynes |
triple bond |
- C Ξ C - |
Halogens |
halogen |
- X ( F,Cl,Br,I ) |
Alcohols |
hydroxyl |
-OH |
Aldehydes |
aldehydic(formyl) |
-CHO |
Carboxylic acids |
carboxyl |
-COOH |
Acid amides Primary amines |
amides amino |
-CONH2 - NH2 |
Homologous series is defined as a family or group of structurally similar organic compounds all members of which contain the same functional group, show a gradation in physical and similarity in chemical properties and any two adjacent members of which differ by -CH2 group. The individual members of this group are called homologues and the phenomenon is called homology.
Organic chemistry deals with millions of compounds. In order to clearly identify them, a systematic method of naming known as IUPAC system of nomenclature is adopted. The names are such that the listener can deduce the structure from it. The IUPAC name consists of three parts:
refix Word root Suffix
EX: 3 meth lyoctane
Straight chain alkanes: The names of such compounds is based on their chain structure , and end with suffix ‘-ane’ and carry a prefix indicating the number of carbon atoms present in the chain.
Branched chain hydrocarbons:
1.) The longest carbon chain in the molecule is identified.
2.) The numbering is done in such a way that the branched carbon atoms get the lowest possible value.
3.) The names of the alkyl groups attached as a branch are then prefixed to the name of the parent alkane and its position is indicated by numbers.
4.) The lower number is given to the first in alphabetical order.
5.) The carbon atom of the branch that attaches to the root alkane is numbered 1.
The longest chain of carbon atoms containing the functional groups is numbered in such a way that the functional group attached to the carbon atom gets the lowest possible number in the chain.
When there are more functional groups then a priority order is followed as:
-COOH, -SO3H, -COOR, COCl, -CONH2, -CN, -HC=O, =C=O, -OH, -NH2, =C=C=, -CΞ C-.
Two or more compounds having the same molecular formula but different physical and chemical properties are called isomers and this phenomenon is called isomerism.
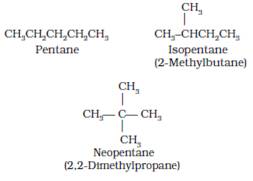
Chain isomerism: When two or more compounds having same molecular formula but different carbon skeletons are referred to as chain isomers.
Position Isomerism : Compounds which have the same structure of carbon chain but differ in position of double or triple bonds or functional group are called position isomers and this phenomenon is called Position Isomerism. e g
CH3-CH2-CH=CH2
CH3-CH = CH – CH3
Functional Isomerism :Compounds which have the same molecular formula but different functional group are called functional isomers and this phenomenon is called functional Isomerism. e g
CH3 – CH2 – OH
CH3 – O – CH3
Metamerism:It is due to the presence of different alkyl groups on either side of functional group in the molecule. Ex. C4H10O represents C2H5OC2H5 and CH3OC3H7.
Heterolytic cleavage: In this cleavage the bond breaks in such a way that the shared pair of electron remains with one of the fragments.
H3C – Br ![]() +CH3 + Br-
+CH3 + Br-
Homolytic Cleavage: In this cleavage the shared pair of electron goes with each of the bonded atom.
R – X ![]() R. + X.
R. + X.
Alkyl free radical
Nucleophiles : A reagent that brings an electron pair is called nucleophile ie nucleus seeking e g -OH , -CN
Electrophiles: A reagent that takes away electron pair is called electrophile I e electron seeking e g C= O , R3C – X
Inductive Effect: The displacement of the electron along the chain of the carbon atoms due to presence of an atom or group at the end of the chain.
d+++ d ++ d+
CH3 ![]() C H2
C H2 ![]() CH2
CH2 ![]() Cl
Cl
Resonance Effect : The polarity produced in the molecule by the interaction of two pi bonds or between a pi bond and lone pair of electron present on an adjacent atom. There are two types of resonance effect:
1) Positive resonance effect : In this effect the transfer of electrons is away from an atom or substituent group attached to the conjugated system.
The atoms or groups which shows +R effect are halogens,-OH , OR,-
NH2
2) Negative resonance effect : In this effect the transfer of electrons is towards the atom or substituent group attached to the conjugated system.
The atoms or groups which shows -R effect are –COOH , -CHO , -CN
Sublimation : This method is used to separate the sublimable compounds from non sublimable compounds.
Crystallisation: This method is based on the difference in the solubilities of compound and impurities in a suitable solvent. The impure compound is dissolved in solvent at heated at higher temp .on cooling the hot and conc solution pure compounds crystallizes out.
Distillation: This method is used to separate volatile liquids from non volatile liquids and liquids having sufficient difference in their boiling points.
Fractional distillation: If the boiling points of two liquids is not much , they are separated by this method.
Distillation under reduced pressure : This method is used to purify liquids having high boiling points and decomposes at or below their boiling points.
Steam distillation : This method is used to separate substances which are steam volatile and are immiscible with water.
Differential Extraction: When an organic compound is present in an aqueous medium it is separated by shaking it with organic solvent in which it is more soluble than in water. The aqueous solution is mixed with organic solvent in a separating funnel and shaken for sometimes and then allowed to stand for some time .when organic solvent and water form two separate layers the lower layer is run out by opening the tap of funnel and organic layer is separated. the process is repeated several times and pure organic compound is separated.
Chromatography :This technique is used to separate mixtures in to their components ,purify the compounds and test the purity of compounds.It is classified as
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.