
p block elements modern periodicttable notes download in pdf, the p-block elements 11 notes, class 11 chemistry notes, the p-block elements class 11, the p-block elements class 11 notes, class 11 the p-block elements, note chemistry, chemistry notes, the p-block elements, class 11 cchemistry chapter 11 notes, 11th standard chemistry notes, 11th std chemistry notes, class 11 chemistry notes chapter 11, the p-block elements chapter class 11 notes
Elements in which the last electron enters in the any one of the three p- orbital of their outermost shells – p-block elements
• Gen. electronic configuration of outer shell is ns2np1-6 The inner core of e-config.may differ which greatly influences their physical & to some extent chemical properties.
• The block of elements in the periodic table consisting of the main groups :
| • Group13 | (B to Tl) |
| • Group14 | (C to Pb) |
| • Group15 | (N to Bi) |
| • Group 16 | (O to Po) |
| • Group17 | (F to At) |
| • Group18 | (He to Rn) |
(1) Members at the top and on the right of the p-block are nonmetals (C, N, P, O, F, S, Cl, Br, I, At).
(2) Those on the left and at the bottom are metals (Al, Ga, In,Tl, Sn, Pb, Sb Bi, Po).
(3) Between the two, from the top left to bottom right, lie an ill-defined group of
metalloid elements (B, Si, Ge, As, Te)
• Outer Electronic Configuration:-ns2np1
• group members: boron (B), aluminum (Al), gallium (Ga), indium (In) & thallium (Tl) . All, except boron, are metals.
• Boron show diagonal relationship with Silicon; both are semiconductors metalloids & forms covalent compounds.
• Boron compounds are electron deficient, they are lack of an octet of electrons about the B atom .
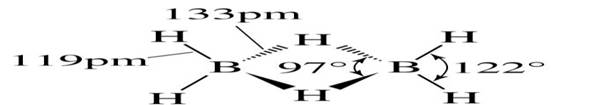
• diborane B2H6 is simplest boron hydride
• Structure: three-center two-electron: the H atoms are simultaneously bonded to two B atoms the B-H bridging bond lengths are greater than B-H terminal.
• - Boron oxide is acidic (it reacts readily with water to form boric acid)
• aluminium compounds:aluminium oxide is amphoteric
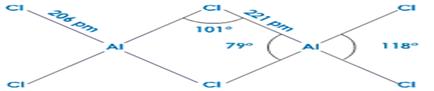
• aluminum halides, e.g., AlCl3 is dimer, an important catalyst in organic chemistry have anincomplete octet, acts as Lewic acid by accepting lone pairs from Lewic bases, forming adduct
• aluminum hydride, e.g., LiAlH4, a reducing agent
• Atomic Properties - Electronic Configurations
Element |
Symbol |
Atomic |
Electronic |
Abundance in Earth’s |
Boron |
B |
5 |
[He]2s2 2p1 |
8 |
Aluminium |
Al |
13 |
[Ne]3s2 3p1 |
81,300 |
Galium |
Ga |
31 |
[Ar]3d104s2 4p1 |
15 |
Indium |
In |
49 |
[Kr] 4d105s2 5p1 |
1 |
Thallium |
Tl |
81 |
[Xe] 5d106s2 6p1 |
0.3 |
• The atomic and ionic radii of group 13 elements are compared to corresponding elements of group 2. From left to right in the period, the magnitude of nuclear charge increases but the electrons are added to, the same shell. These electrons do not screen each other, therefore, the electrons experience greater nuclear charge.
• In other words, effective nuclear charge increases and thus, size decreases. Therefore, the elements of this group have smaller size than the corresponding elements of second group.
• On moving down the group both atomic and ionic radii are expected to increase due to the addition of new shells. However, the observed atomic radius of Al (143 pm) is slightly more than that of Ga (l35 pm).
The first ionization energies of group 13 elements are less than the corresponding members of the alkaline earths.
The sharp decrease in I.E. from B to Al is due to increase in size. In case of Ga, there are ten d-electrons in its inner electronic configuration.
The very high value of 3rd I. E. of thallium indicates that +3 O.N. state is not stable, rather +1 is more stable for thallium .
the elements of group 13 are less electropositive as compared to elements of group 2. On moving down the group the electropositive (metallic) character increases because ionization energy decreases. For e.g., Boron is a non-metal white the other elements are typical metals.
The common oxidation states of group 13 elements are +3 and + l .The stability of the + 1 oxidation state increases in the sequence Al <Ga< In <Tl, Due to Inert pair effect.
Element |
B |
Al |
Ga |
In |
Tl |
Oxidation state |
+3 |
+3 |
+3, +1 |
+3, +1 |
+3, +1 |
All elements in their compounds exhibit the oxidation state of + 3 and +1. Hydrides
• None of the group 13 elements reacts directly with hydrogen. However, a no.
of hydrides of these elements have been prepared by indirect methods. The boron hydrides are called boranes& classified in two series: (a) BnHn+4 called nidoboranes (b) BnHn+6 called arachnoboranes
2BF3(g) + 6LiH(s) → B2H6(g) + 6LiF(s)
• Laboratory method:
(i) By the reaction of iodine with sodium borohydride in a high boiling solvent. 2NaBH4 + I2 → B2H6 + 2NaI + H2
(ii) By reduction of BCl3 with LiAlH4 4BCl3 + 3LiAlH4 → 2 B2H6 + 3AlCl3 + 3 LiCl
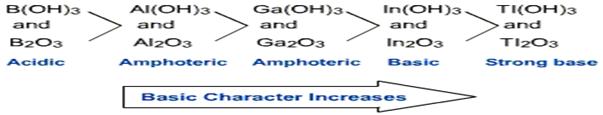
Some important characteristics of boranes:
i) Lower boranes are colourless gases while higher boranes are volatile liquids or solids.
ii) They undergo spontaneous combustion in air due to strong affinity of boron for oxygen. B 2H6 + 3O2 → B2O3 + 3H2O + Heat
iii) Boranes react with alkali metal hydrides in diethyl ether to form borohydride complexes. B2H6 + 2MH →2M+[BH4]- (M= Li or Na) Metal borohydride
(iv) Diborane reacts with ammonia to give borazine at 450 K. B2H6 + 6NH3 → 3B3N3H6 + 12H2
• Borazine has a cyclic structure similar to benzene and thus is called inorganic benzene
• The other elements of this group form only a few stable hydrides. The thermal stability decreases as we move down the group.
• AlH3 is a colourless solid polymerized via Al - H - Al bridging units. These hydrides are weak Lewis acids and readily form adducts with strong Lewis base (B:) to give compounds of the type MH3 (M = Al or Ga). They also form complex-tetrahydrido anions, [MH4]-. The most important tetrahydrido compound is Li[AlH4]
![tetrahydrido compound is Li[AlH4]](ncertimages/class 11/chemistry/CH 11/tetrahydrido compound is Li[AlH4]jpg.jpg)

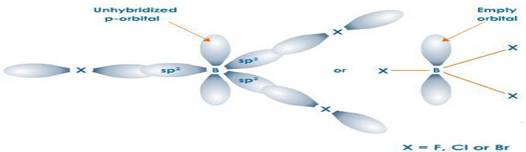
– Boron halides do not form dimers because the size of boron is so small that it is unable to coordinate four large-sized halide ions.
1. Boron is a non-metal & bad conductor of electricity whereas aluminium is a metal& good conductor. B is hard but Al is a soft metal.
2. Boron exists in two forms-crystalline and amorphous. But Al does not exist in different forms.
3. The melting and boiling point of boron are much higher than that of Al .
4. Boron forms only covalent compounds whereas Al forms even some ionic compounds.
5. The hydroxides and oxides of boron are acidic in nature whereas those of aluminium are amphoteric.
6. The trihalides of boron exist as monomers. On the other hand, aluminium halides
exist as dimers .
7. The hydrides of boron are quite stable while those of aluminium are unstable
• Boron and silicon exhibit the typical properties of non-metals. These do not form cations. Both exist in amorphous as well as crystalline forms.
• Boron oxide (B2O3) and silic a (SiO2) both are acidic and dissolve in alkali solutions to form borates and silicates respectively. B2O3 + 6NaOH → 2Na2BO3 + 3H2O SiO2 + 2NaOH → Na2SiO3 + H2O
• The chlorides of both B and Si get hydrolyzed by water to boric acid and silicic acid respectively. BCl3 + 3H2O →H3BO3 + 3HCl SiCl4 + 3H2O → H2SiO3 + 4HCl
The hydrides of Boron and Silicon are quite stable. Numerous volatile hydrides are also known which catch fire on exposure to air and are easily hydrolyzed. Both elements are semiconductors.
1 Al, Ga, In and Tl exhibit a well-defined aqueous chemistry in their tripositive states. Species like [M(OH)4]-, [M(H2O)2(OH)4]-, [M(OH2)6]3+ for M = Al, Ga, In, exist in aqueous solution.
2. Al, Ga. In and T1 ions exist as octahedral aqua ions, [M(OH2)6]3 + in aqueous solution and many salts like halides, sulphates, nitrates and perchlorates exist as hydrates.
3. Aluminiumsulphate forms double salts - called alum, having the general formula M2SO4. Al2(SO4)3.12H2O, where M=Na+ or K+. USES OF BORON & ALUMINIUM
• Aluminium is used extensively in industry and everyday life. It forms many useful alloys with Cu. Mn, Mg, Si and Zn. Hence, aluminium and its alloys find use in packaging, utensil making, construction, aerospace and other transportation industries. It is used as a conductor for transmission of electricity. Aluminium is alsoused in the alumino-thermite process for production of chromium and manganese from their ores.
Group 14 includes carbon (C), silicon (Si), germanium (Ge), tin (Sn) and lead (Pb). General electronic configuration of carbon family is ns2np2. Covalent radius:-Covalent radius expected to increase from Cto Si, From Si to Pb small increase is found.
Ionization Enthalpy:-The first ionization enthalpies of group 14 elements are higher than those of the corresponding group 13 elements. Electronegativity:-Group 14 elements are smaller in size as compared to group 13 elements that’s why this group elements are slightly more electronegative than group13
Carbon and silicon mostly show +4 oxidation state. Germanium forms stable compounds in +4 state and only few compounds in +2 state.
Tin forms compounds in both oxidation states. Lead compounds in +2 state are stable and in +4 state are strong oxidizing agents.
Exception:-Pb4 and SnF4 are ionic in nature.
Except CCl4 other tetrachlorides are easily hydrolysed by water.
Since carbon does not have d-orbitals and hence cannot expand its coordination number beyond 4
CCl4 +H2O No Reaction
SiCl4+4H2O Si(OH)4+4HCl Silicic acid
1-Diamond
2-Graphite
3-Fullerence
In diamond each carbon atom undergas SP3hybridisation. Each carbon is tetrahedrally linked to four other carbon atoms.
In graphite, carbon is SP2-hyberdized graphite has a two-dimensional sheet like structure consisting of a number of hexagonal rings fused together. Graphite conducts electricity along the sheet.It is very soft and Slippery FullerenceFullerence was discovered collectively by three scientists namely R.E Smalley,R.F Curl and H.W Kroto SOME Important Compounds Of Carbon and Silicon
It I prepared by direct oxdisation of C in limited supply of oxygen. 2C+O2(g) → 2CO(g Commercially it is prepared by the passage of steam over hot coke
It is prepared by complete combustion of carbon and carbon fuels in excess of air.C(s) +O2(g) → CO2(g)
In laboratory it is prepared by the treatment of dilHCl on CaCO3 CaCO3(s) +2HCl(aq) → CaCl2(aq) +CO2>/(g)+H2O(l) Silicon
Silicon dioxide is a COVALENT THREE DIMENSIONAL NETWORK SOLID.
Each silicon atom is covalently bonded in a tetrahedral manner to four oxygen atoms. Silicones:-Silicones are the synthetic organo-siliconpolymers having general formulae (R2SiO)n in which R = alkyl (methyl,ethyl or phenyl) Silicates:-Silicates are exist in nature in the form of feldspar, zeolites,mica and asbestos etc. The basic structure of silicates is SiO44
-Zeolites is aalumino-silicate of metal. Metal cations participating in formationof Zeolite are use usually Na+,K+,or Ca2+. Zeolites are used to remove permanent hardness of water.
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.