
organic chemistry, chemistry software, chemistry encyclopedia, chemistry xi, chemistry for dummies, chemical bonding and molecular structure 11 notes, class 11 chemistry notes, chemical bonding and molecular structure class 11, chemical bonding and molecular structure class 11 notes, class 11 chemical bonding and molecular structure, note chemistry, chemistry notes, chemical bonding and molecular structure, class 11 cchemistry chapter 4 notes, 11th standard chemistry notes, 11th std chemistry notes, class 11 chemistry notes chapter 4, chemical bonding and molecular structure chapter class 11 notes
During a chemical reaction the atoms tend to adjust their
electronic arrangement in such a way that they achieve 8 e- in their outermost electron. This is called octet rule.
the chemical force which keeps the atoms in any molecule together is called a chemical bond.
The columbic force of attraction which holds the appositively charged ions together is called an ionic bond. An ionic bond is formed by the complete transfer of one or more electrons from the atom of a metal to an atom of non- metal.
The molar enthalpy change accompanying the complete separation of the constituent particles that compose of the solids (such as ions for ionic solid, molecules for molecular solids) under standard conditions is called lattice enthalpy (∆lHo). The lattice enthalpy is a positive quantity.
The number of electrons lost or gain by an atom of an element is called as electrovalency.The element which give up electrons to form positive ions are said to have positive valency, while the elements which accept electrons to form negative ions are said to have negative valency.
It is favoured by
(i) the low ionisation enthalpy of a metallic element which forms the cations,
(ii) High electron gain enthalpy of non- metallic element which forms the anions,
(iii) Large lattice enthalpy i.e; the smaller size and the higher charge of the atoms.
The number of electrons which an atom contributes towards mutual sharing during the formation of a chemical bond called its covalency in that compound.
A covalent bond formed by the mutual sharing of one pair of electrons is called a single covalent bond, or simply a single bond. A single covalent bond is represented by a small line (−) between the two atoms.
A covalent bond formed by the mutual sharing of two pair of electrons is called a double covalent bond, or simply a double bond. A double covalent bond is represented by two small horizontal lines (=) between the two atoms. E.g. O=O, O=C=O etc.
A covalent bond formed by the mutual sharing of three pair of electrons is called a triple covalent bond, or simply a triple bond. A triple covalent bond is represented by three small horizontal lines (≡) between the two atoms. E.g. N≡N, H-C≡C-H etc.
Formation of a covalent bond is favoured by
(i) High ionisation enthalpy of the combining elements.
(ii) Nearly equal electron gain enthalpy and equal electro-negativities of combining elements.
(iii) High nuclear charge and small atomic size of the combining elements.
The bond between two unlike atoms which differ in their affinities for electrons is said to be polar covalent bond. E.g. H-Cl
The bond formed when one sided sharing of electrons take place is called a coordinate bond. Such a bond is also known as dative bond. It is represented by an arrow (→) pointing towards the acceptor atom. E.g. H3N→BF3
Bond Length : Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule
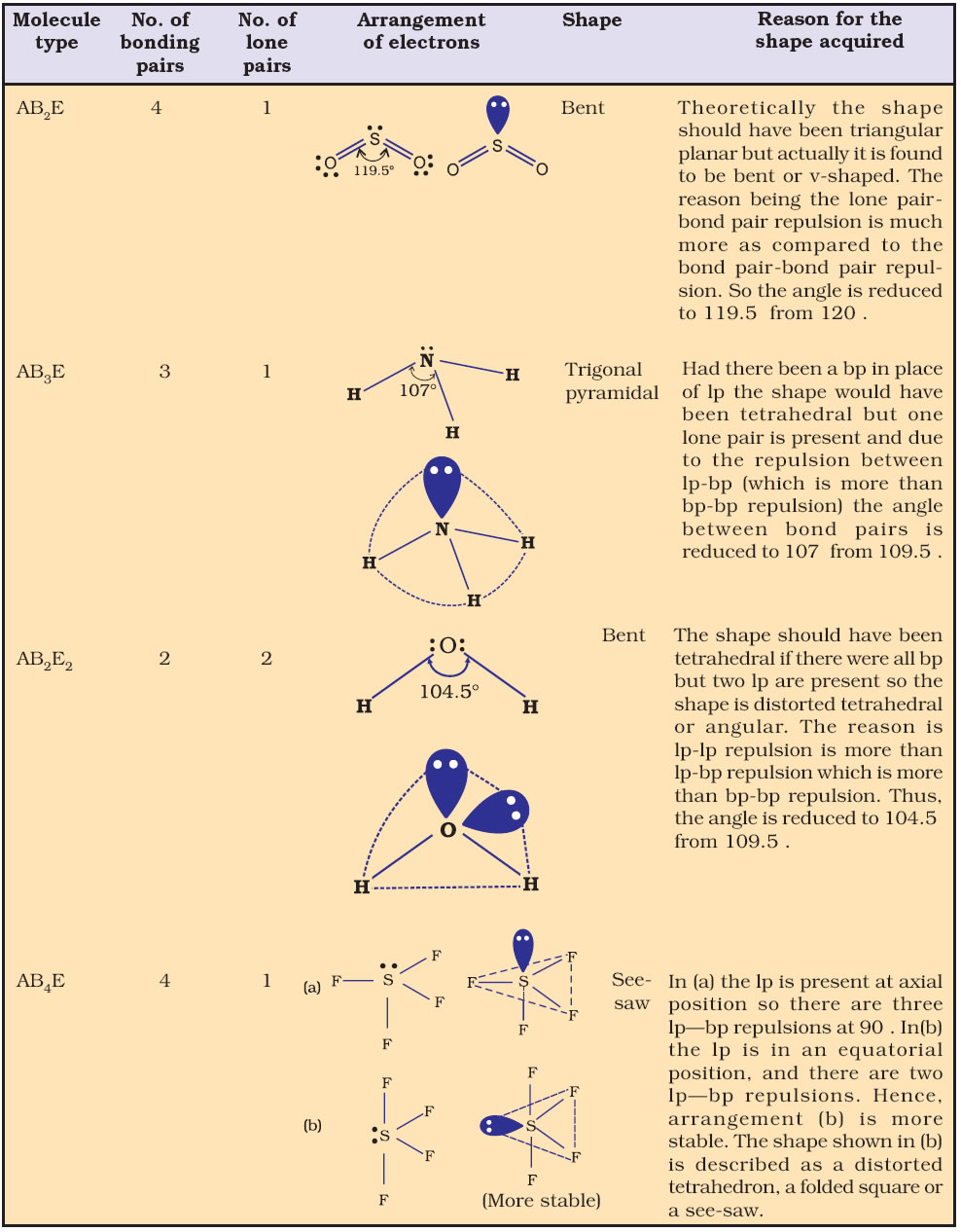
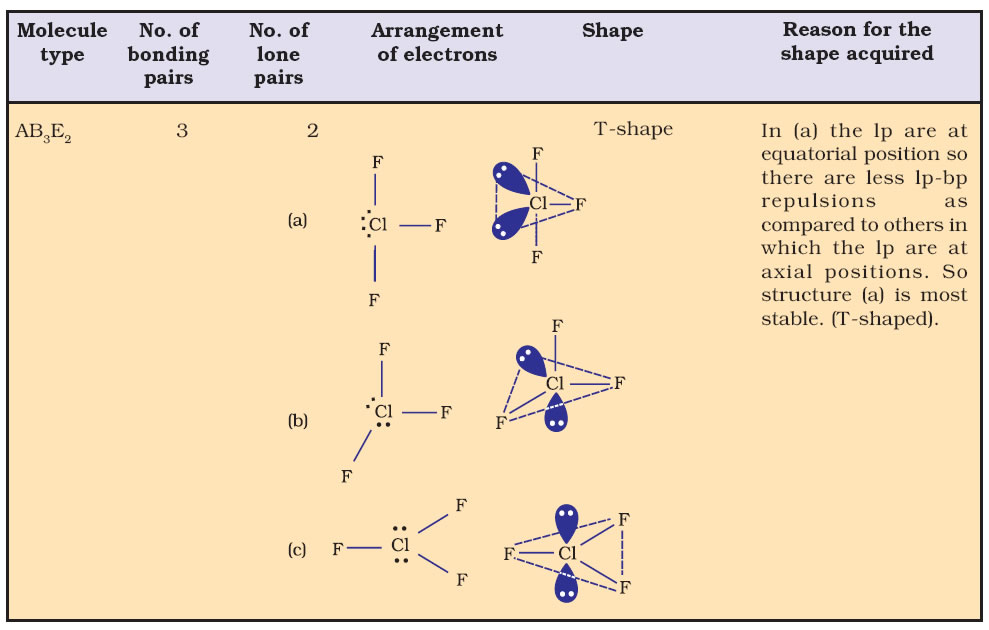
Bond Angle : It is defined as the angle between the orbitals containing bonding electron pairs around the central atom in a molecule/complex ion
Bond Enthalpy : It is defined as the amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state.
Bond Order : In the Lewis description of covalent bond, the Bond Order is given by the number of ondsetween the two atoms in a molecule
Resonance : whenever a single Lewis structure cannot describe a molecule
accurately, a number of tructures with similar energy, positions of nuclei, bonding and non-bonding pairs of electrons are taken as he canonical structures of the
hybrid which describes the molecule accurately
Dipole moment : The product of the magnitude of the charge and the distance between the centres of positive and negative charge.It is a vector quantity and is represented by an arrow with its tail at the positive centre and head pointing towards a negative centre. Dipole moment (μ) = charge (Q) × distance of separation (r)
SIGMA BOND : A covalent bond formed due to the overlapping of orbitals of the two atoms along the line joining the two nuclei (orbital axis) is called sigma (σ) bond. For example, the bond formed due to s-s and s-p, p-p overlapping along the orbital axis are sigma bonds.
A covalent bond formed by the side wise overlapping of p- or d- orbitals of two atoms is called as pi (π) bond. For example, the bond formed due to the sideways overlapping of the two p- orbitals is a pi- bond.
The bond between the hydrogen atom of one molecule and
a more electro- negative element of same or another molecule is called as hydrogen bond.
The process of mixing of the atomic orbitals to form new hybrid orbitals is called hybridization. All hybrid orbitals of a particular kind have equal energy, identical shapes and are symmetrically oriented in shape. The hybrid orbitals are designed according to the type and the atomic orbitals merging together, e.g.,
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.