
7 states of matter, 5 states of matter, different states of matter, five states of matter, state of matter, fifth state of matter, 6th state of matter, states of matter ppt, interconversion of states of matter, sixth state of matter, states of matter wikipedia, how many states of matter, four states of matter, 12 states of matter, , states of matter 11 notes, class 11 chemistry notes, states of matter class 11, states of matter class 11 notes, class 11 states of matter, note chemistry, chemistry notes, states of matter, class 11 cchemistry chapter 5 notes, 11th standard chemistry notes, 11th std chemistry notes, class 11 chemistry notes chapter 5, states of matter chapter class 11 notes
1. Change in state : It is over all effect of Intermolecular forces, molecular Interactional energy & thermal energy:
2. Measurable properties of gases : P,V, T, n, Viscosity, specific heat are some measurable properties.
3. Gas Laws : The quantitative relationship b/w any two of the variables (V, n, P,T) when other two are constant.
4. Boyle’s Law : The pressure of fixed msss of gas varies inversely with the volume at constant T. P α 1/V(n,T const.) P1V1 = 2V 2
5. Charle’s Law : At constant P, the volume of fixed amount of gas varies directly with its absolute temperature.
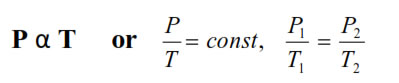
6. Gay lussac’s Law : At constant V, The pressure of fixedamount of gas varies directly with its absolute temperature.
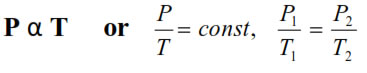
7. Ideal gas equation : The relationship b/w P, V and T by Gas Laws PV= nRT.
8. Avogadro’s Law : At given T and P, the volume of gasvaries directly to the amount of gas . V α n ( P, T constant)
9. Dalton’s Law of partial persure : The pressure enerted by a mixture of non reacting gases is equal to the sum of their partial pressure at constant (V,T)P (total ) = P1 + P2 + P3 + ………. (T, V, constant)
10. Kinetic Molecular theory :
a. Gases consist of large number of identical particles (atoms or molecules) that are so small that the actual volume of the molecules is negligible in comparison to the empty space between them.
b. There is no force of attraction between the particles of a gas at ordinary temperature and pressure
c. Particles of a gas are always in constant and random motion
d. Pressure is exerted by the gas as a result of collision of the particles with the walls of the container
e. Collisions of gas molecules are perfectly elastic
f. At any particular time, different particles in the gas have different speeds and hence different kinetic energies
g. Average kinetic energy of the gas molecules is directly proportional to the absolute temperature
11. Real Gases : The gases which deviates from Ideal behavior at higher pressure and low temperature b/c of force of attraction b/w molecules increases .
12. Compressibility factor (Z) : It determine extent of devation of real gases from Ideal gas behavior
![]() for ideal gass formula gass Z=1, for Nonideal gas Z< 1, Z > 1
for ideal gass formula gass Z=1, for Nonideal gas Z< 1, Z > 1
Vandor Walls equations:- The constants a and b have positive values and are characteristic of the individual gas. The van der Waals equation of state approaches the ideal gas law
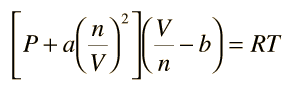
14. Critical Temperature : (Tc) The temperature above which a gas cannot be liquefied whatever high pressure may be
15. Critical Pressure : The minimum pressure required to liquity a gas at its critical temperature.
16. Critical Volume : The volume of 1 mole of gat at Tc, Pc.
17. Super cooled liquids : The liquids which are cooled to a temperature below its freezing point without freezing .
18. Elastic Collision : The collisions in which no loss of K.E. only there is transfer of energy.
19. Vapour pressure : The equilibrium pressure by vapour of liquid in a container at given temperature (T)
20. At higher altitude : The b.p. of water decreases b/c the atmospheric pressure is less than one atmosphere.
21. Surface Tension (V) : It is force acting per unit length perpendicular to the line drawn on the surface : (Nm-1) : It decreases with increases in T, it increases with increase in external pressure, b/c of it falling drops of liquid are spherical, liquid in capillary tube rises.
22. Viscosity (η) : It is resistance offered to the flow of liquid
23. Effect of T & P on viscosity : It decreases with increase inT, and increases with increase in P.
24. Low M.P. & B.P. of molecular liquids is due to low magnitude of molecular interaction energy.
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.