
class 11 chemistry notes, 11 class chemistry notes, class 12 chemistry notes, chemistry class 11 notes, chemistry notes, chemistry notes for class 11, class 10 chemistry notes, 12 class chemistry notes, chemistry notes for class 12, some basic concepts of chemistry 11 notes, class 11 chemistry notes, some basic concepts of chemistry class 11, some basic concepts of chemistry class 11 notes, class 11 some basic concepts of chemistry, note chemistry, chemistry notes, some basic concepts of chemistry, class 11 cchemistry chapter 1 notes, 11th standard chemistry notes, 11th std chemistry notes, class 11 chemistry notes chapter 1, some basic concepts of chemistry chapter class 11 notes
SOME BASIC CONCEPTS OF CHEMISTRY
Chapter 1 Class 11 Download in pdf
Every substance has unique or characteristic properties. These properties can be classified into two categories – physical properties and chemical properties.
Physical properties are those properties which can be measured or observed without changing the identity or the composition of the substance. E.g. colour, odour, melting point, boiling point, density etc.The measurement or observation of chemical properties requires a chemical change occur. e.g. Burning of Mg-ribbon in air
Chemical properties are characteristic reactions of different substances; these include acidity or basicity, combustibility etc.Many properties of matter such as length, area, volume, etc., are quantitative in nature.
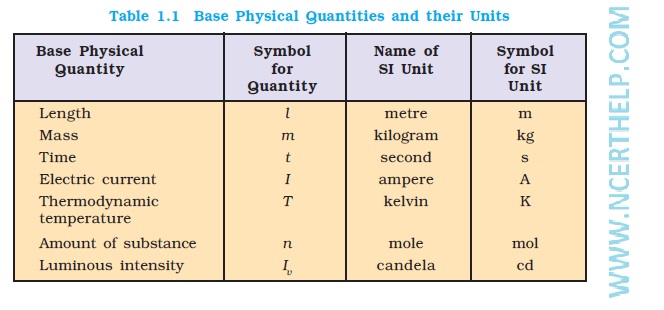
The International System of Units (in French Le Systeme International d’Unites– abbreviated as SI) was established by the 11th General Conference on Weights and Measures (CGPM fromConferenceGenerale des Poids at Measures). The SI system has seven base units
Metric System was based on the decimal system.
Mass and Weight- Mass of a substance is the amount of matter present in it while weight is the force exerted by gravity on an object. The mass of a substance is constant whereas its weight may vary from one place to another due to change in gravity. The mass of a substance can be determined very accurately by using an analytical balance
Volume- Volume has the units of (length)3. So volume has units of m3 or cm3 or dm3.A common unit, litre (L) is not an SI unit, is used for measurement of volume of liquids. 1 L = 1000 mL, 1000 cm3 = 1 dm3
Density- Density of a substance is its amount of mass per unit volume.SI unit of density = SI unit of mass/SI unit of volume = kg/m3 or kg m–3This unit is quite large and a chemist often expresses density in g cm–3.
Temperature-There are three common scales to measure emperature — °C (degree celsius), °F (degree Fahrenheit) and K (kelvin). Here, K is the SI unit.
![]()
K = °C + 273.15
Note- Temperature below 0 °C (i.e. negative values) are possible in Celsius scale but in Kelvin scale, negative temperature is not possible.
Scientific Notation- In which any number can be represented in the form N × 10n Where n is an exponent having positive or negative values and N can vary between 1 to 10). e.g. We can write 232.508 as 2.32508 x102 in scientific notation. Similarly, 0.00016 can be written as 1.6 x 10–4.
Precision refers to the closeness of various measurements for the same quantity.
Accuracy is the agreement of a particular value to the true value of the result
Significant Figures
The reliability of a measurement is indicated by the number of digits used to
represent it. To express it more accuratelywe express it with digits that are known with certainty. These are called as Significant figures. They contain all thecertain digits plus one doubtful digit in a number.
The rounding off procedure is applied to retain the required number of significant figures.
Dimensional Analysis During calculations generally there is a need to convert units from one system to other. This is called factor label method or unit factor method or dimensional analysis.
For example- 5 feet and 2 inches (height of an Indian female) is to converted in SI unit
1 inch = 2.54 x 10-2 m
then, 5 feet and 2 inch = 62 inch
Properties |
Solid |
Liquid |
Gas |
1. volume |
Definite |
Definite |
Indefinite |
2. Shape |
Definite |
Indefinite |
Indefinite |
3. Inter molecular |
Very high |
Moderate |
Negligible / Very |
4. arrangement of |
Orderly arranged |
Free to move |
Free to move every |
5. Inter molecular |
Very small |
Slightly greater |
Very great |
7. Compressibility |
Not compressible |
Not compressible |
Highly |
8. Expansion on |
Very little |
Very little |
Highly expand |
9. Rigidity |
Very rigid |
Not rigid knownas |
Not rigid and |
9. Fluidity |
Can’t flow |
Can flow |
Can flow |
10. Diffusion |
They can diffuse |
Can diffuse And |
Can diffuse And |
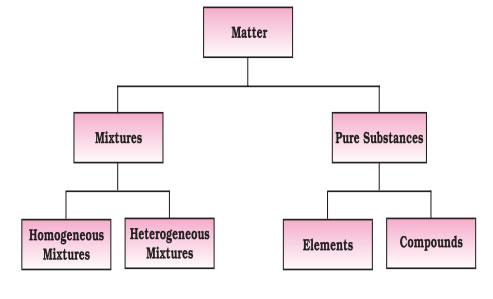
An element is the simplest form of matter that cannot be split into simpler substances
or built from simpler substances by any ordinary chemical or physical method. There are 114 elements known to us, out of which 92 are naturally occurring while the rest
have been prepared artificially.
Elements are further classified into metals, non-metals and metalloids.
A compound is a pure substance made up of two or more elements combined in a definite proportion by mass, which could be split by suitable chemical methods. Compounds are broadly classified into inorganic and organic compounds. Inorganic compounds are those, which areobtained from non-living sources such as minerals. For example, common salt, marble and limestone. Organiccompounds are those, which occur in living sources such as plants and animals. They all contain carbon. Commonorganic compounds are oils, wax, fats etc.
Characteristics of compound
A mixture is a combination of two or more elements or compounds in any proportion so that the components do not lose their identity. Air is an example of a mixture Mixtures are of two types, homogeneous and heterogeneous.
Heterogeneous mixtures - have the same composition throughout the sample. The components of such mixtures cannot be seen under a powerful microscope. They are also called solutions. Examples of homogeneous mixtures are air, seawater, gasoline, brass etc.Law of Conservation of Mass (Given by Antoine Lavoisier in 1789). It states that matter (mass) can neither be created nor destroyed.
Law of Definite Proportions or Law of Constant Composition: This law was proposed by Louis Proust in 1799, which states that: ’A chemical compound always consists of the same elements combined together in the same ratio, irrespective of the method of preparation or the source from where it is taken’.
Law of Multiple Proportions Proposed by Dalton in 1803, this law states that:’ When two elements combine to form two or more compounds, then the differe nt masses of one element, which combine with a fixed mass of the other, bear a simple ratio to one another’.
Gay Lussac’s Law of Gaseous Volumes (Given by Gay Lussac in 1808.) According to this law when gases combine or are produced in a chemical reaction they do so in a simple ratio by volume provided all gases are at same temperature and pressure.
Example
| H2(g) + | + | Cl2(g) | → | 2HCl(g) |
|---|---|---|---|---|
| 1V | 1V | 2V |
All reactants and products have simple ratio 1:1:2
Avogadro Law (In 1811, Given by Avogadro)
According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules.
Atoms and Molecules
The smallest particle of an element, which may or may not have independent
existence is called an atom, while the smallest particle of a substance which is capable of independent existence is called a molecule.
Molecules are classified as homoatomic and heteroatomic. Homoatomic molecules are made up of the atoms of the same element and heteroatomic molecules are made
up of the atoms of the different element have different atomicity (number of atoms in a molecule of an element) like monoatomic, diatomic, triatomic and polyatomic.
Atomic Mass Unit
One atomic mass unit is defined as a mass exactly equal to one twelfth the mass of one carbon -12 atom. And 1 amu = 1.66056×10–24 g.
Today, ‘amu’ has been replaced by ‘u’ which is known as unified mass.
Atomic Mass
Atomic mass of an element is defined as the average relative mass of an atom of an element as compared to the mass of an atom of carbon -12 taken as 12.
![]()
Gram Atomic Mass
The quantity of an element whose mass in grams is numerically equal to its atomic mass. In simple terms, atomic mass of an element expressed in grams is the gram atomic mass or gram atom. For example, the atomic mass of oxygen = 16 amu Therefore gram atomic mass of oxygen = 16 g
Molecular Mass
Molecular mass of a substance is defined as the average relative mass of its molecule
as compared to the mass of an atom of C-12 taken as 12. It expresses as to how many times the molecule of a substance is heavier than 1/12th of the mass of an atom of carbon.
For example, a molecule of carbon dioxide is 44 times heavier than 1/12th of the mass of an atom of carbon. Therefore the molecular mass of CO2 is 44 amu.
It is obtained by adding the atomic masses of all the atoms present in one molecule.
Gram Molecular Mass
A quantity of substance whose mass in grams is numerically equal to its molecular
mass is called gram molecular mass. In simple terms, molecular mass of a substance expressed in grams is called gram molecular mass.
e.g., the molecular mass of oxygen = 32 amu
Therefore, gram molecular mass of oxygen = 32 g
Formula Mass-
Sum of atomic masses of the elements present in one formula unit of a compound. It is used for the ionic compounds.
Mole Concept - Mole is defined as the amount of a substance, which contains the same number of chemical units (atoms, molecules, ions or electrons) as there are atoms in exactly 12 grams of pure carbon-12.
A mole represents a collection of 6.022 x1023( Avogadro’s number) chemical units..The mass of one mole of a substance in grams is called its molar mass.
Molar Volume
The volume occupied by one mole of any substance is called its molar volume. It is
denoted by Vm. One mole of all gaseous substances at 273 K and 1 atm pressure occupies a volume equal to 22.4 litre or 22,400 mL. The unit of molar volume is litre
per mol or millilitre per mol
PERCENTAGE COMPOSITION—
The mass percentage of each constituent element present in any compound is called
its percentage composition
Mass % of the element= (Mass of element in 1 molecule of the compound x100) /
Molecular mass of the compound
An empirical formula represents the simplest whole number ratio of various atoms present in a compound. E.g. CH is the empirical formula of benzene.
The molecular formula shows the exact number of different types of atoms present in a molecule of a compound. E.g. C6H6 is the molecular formula of benzene.
Relationship between empirical and molecular formulae
The two formulas are related as Molecular formula = n x empirical formula
![]()
Shorthand representation of a chemical change in terms ofsymbols and formulae of
the substances involved in the reaction is called chemical equation.
The substances that react among themselves to bring about the chemical changes are known as reactants, whereas the substances that are produced as a result of the
chemical change, are known as products
Limiting Reagent- The reactant which gets consumed first or limits the amount of product formed is known as limiting reagent
Reactions in Solutions-- The concentration of a solution can be expressed in any of
the following ways.
1. Mass Percent is the mass of the solute in grams per 100 grams of the solution.
![]()
2. Volume percent is the number of units of volume of the solute per 100 units of the volume of solution.
![]()
A 5 % (v/v) solution of ethyl alcohol contains 5 cm3 of alcohol in 100 cm3 of the solution
3. Molarity of the solution is defined as the number of moles of solute dissolved per litre (dm3) of the solution. It isdenoted by the symbol M. Measurements in Molarity can change with the change in temperature because solutionsexpand or contract accordingly.
Molarity of the solution = Mass of the solute/(
Molar mass of the solute X volume of the solution in liter)
Formula :-
Where
W= Mass of the solute
M=
Molar mass of the solute
V= volume of the solution in liter)
Molarity equation- To calculate the volume of a definite solution required to prepare solution of other molarity, the following equation is used: M1V1 = M2V2,
where M1= initial molarity, M2= molarity of the new solution, V1= initial volume and V2= volume of the new solution.
4. Molality- Molality is defined as the number of moles of solute dissolved per 1000 g (1 kg) of solvent. Molality is expressed as ’m’.
![]()
5. Mole Fraction is the ratio of number of moles of one component to the total number of moles (solute and solvents) present in the solution. It is expressed as’x’.
Mole fraction of the solute = Moles of the solute/( Moles of solute + Moles of solvent)
Mole fraction of the solvent =Moles of the solvent/( Moles of solute + Moles of solvent)
Always Check
Mole fraction of the solute + Mole fraction of solvent = 1
Please send your queries to ncerthelp@gmail.com you can aslo visit our facebook page to get quick help. Link of our facebook page is given in sidebar
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.