Explain type of cells ?
In the primary batteries, the reaction occurs only once and after use over a period of time battery becomes dead and cannot be reused again.
Example
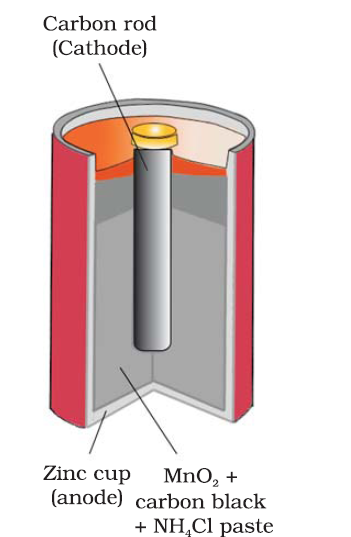
Leclanche cell : The cell consists of a zinc container that also acts as anode and the cathode is a carbon (graphite) rod surrounded by powdered manganese dioxide and carbon (Fig.3.8). The space between the electrodes is filled by a moist paste of ammonium chloride (NH4Cl) and zinc chloride (ZnCl2).
Anode: Zn(s) → Zn2+ + 2e-
Cathode: MnO2+ NH4+ + e- → MnO(OH) + NH3
The cell has a potential of nearly 1.5 V.
Use : write your self
Mercury cell
consists of zinc – mercury amalgam as anode and a paste of HgO and carbon as the cathode. The electrolyte is a paste of KOH and ZnO. The electrode reactions for the cell are given below:
Anode: Zn(Hg) + 2OH- → ZnO(s) + H2O + 2e-
Cathode: HgO + H2O + 2e- → Hg(l ) + 2OH-
The overall reaction is represented by
Zn(Hg) + HgO(s) → ZnO(s) + Hg(l )
The cell potential is approximately 1.35 V and remains constant during its life as the overall
Use :- write your self
Secondary Batteries
A secondary cell after use can be recharged by passing current through it in the opposite direction so that it can be used again.
Example
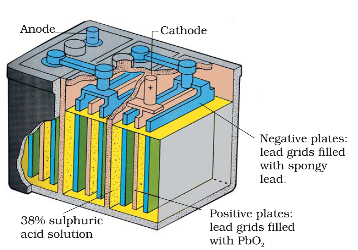
Lead storage battery
It consists of a lead anode and a grid of lead packed with lead dioxide (PbO2 ) as cathode. A 38% solution of sulphuric acid is used as an electrolyte. The cell reactions when the battery is in use are given below:
Anode: Pb(s) + SO42–(aq) → PbSO4(s) + 2e–
Cathode: PbO2(s) + SO42–(aq) + 4H+(aq) + 2e– → PbSO4 (s) + 2H2O (l )
i.e., overall cell reaction consisting of cathode and anode reactions is:
Pb(s)+PbO2(s)+2H2SO4(aq)→ 2PbSO4(s) + 2H2O(l)
On charging the battery the reaction is reversed and PbSO4(s) on
anode and cathode is converted into Pb and PbO2, respectively.
Nickel cadmium cell
longer life than the lead storage cell but more expensive to manufacture overall reaction during discharge is:
Cd (s)+2Ni(OH)3 (s) → CdO (s) +2Ni(OH)2 (s) +H2O(l )
Write mechanism or advantages of hydrogen cell?
In fuel Cellshydrogen and oxygen are bubbled through porous carbon electrodes into concentrated aqueous sodium hydroxide solution. Catalysts like finely divided platinum or palladium metal are incorporated into the electrodes for increasing the rate of electrode reactions. The electrode reactions are given below :
Cathode: O2(g) + 2H2O(l ) + 4e- → 4OH-(aq)
Anode: 2H2 (g) + 4OH-(aq) → 4H2O(l) + 4e-
Overall reaction being:
2H2(g) + O2(g) → 2 H2O(l )
Advantages
(1) The cell runs continuously as long as the reactants are supplied.
(2) Fuel cells produce electricity with an efficiency of about 70 % compared to thermal plants whose efficiency is about 40%.
(3) Fuel cells are pollution free and in view of their future importance
(4) The water vapours produced during the reaction were condensed and added to the drinking water supply