
alcohols, phenols and ethers class 12 ncert solutions, , ncert solutions, chapter 11,chapter 11ncert solutions, alcohols, phenols and ethers ncert solutions, ncert solutions for class 12 chemistry, class 12 chemistry ncert solutions, ncert solutions for class 12, ncert class 12 chemistry, class 12 chemistry, class 12 chemistry solution, ncert solutions class 12, class 12 chemistry , ncert class 12, class 12 chemistry chapter 11,chapter 11 alcohols, phenols and ethers ncert solutions
Question 11.2 Write structures of the compounds whose IUPAC names are as follows:
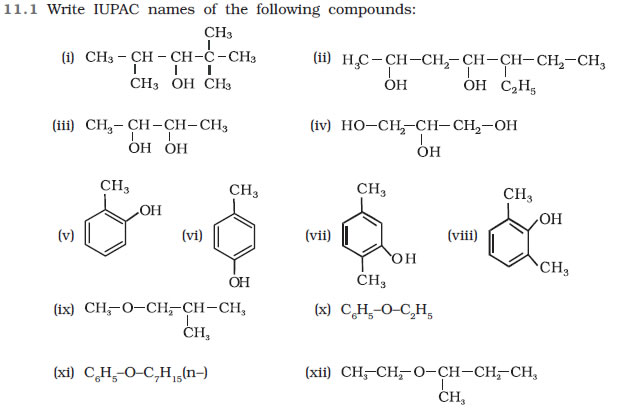
(i) 2-Methylbutan-2-ol
(ii) 1-Phenylpropan-2-ol
(iii) 3,5-Dimethylhexane –1, 3, 5-triol (iv) 2,3 – Diethylphenol
(v) 1 – Ethoxypropane
(vi) 2-Ethoxy-3-methylpentane
(vii) Cyclohexylmethanol
(viii) 3-Cyclohexylpentan-3-ol
(ix) Cyclopent-3-en-1-ol
(x) 3-Chloromethylpentan-1-ol.
1
Question 11..3 (i) Draw the structures of all isomeric alcohols of molecular formula C5H12O
and give their IUPAC names.
(ii) Classify the isomers of alcohols in question
Question 11.3 (i) as primary, secondary
and tertiary alcohols.
Question 11.4 Explain why propanol has higher boiling point than that of the hydrocarbon,
butane?
Question 11.5 Alcohols are comparatively more soluble in water than hydrocarbons of
comparable molecular masses. Explain this fact.
Question 11.6 What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Question 11.7 Give the structures and IUPAC names of monohydric phenols of molecular
formula, C7H8O.
Question 11.8 While separating a mixture of ortho and para nitrophenols by steam
distillation, name the isomer which will be steam volatile. Give reason.
Question 11.9 Give the equations of reactions for the preparation of phenol from cumene.
Question 11.10 Write chemical reaction for the preparation of phenol from chlorobenzene.
Question 11.11 Write the mechanism of hydration of ethene to yield ethanol.
Question 11.12 You are given benzene, conc. H2SO4 and NaOH. Write the equations for the
preparation of phenol using these reagents.
Question 11.13 Show how will you synthesise:
(i) 1-phenylethanol from a suitable alkene
.
(ii) cyclohexylmethanol using an alkyl halide by an SN2 reaction.
(iii) pentan-1-ol using a suitable alkyl halide?
Question 11.14 Give two reactions that show the acidic nature of phenol. Compare acidity
of phenol with that of ethanol.
Question 11.15 Explain why is ortho nitrophenol more acidic than ortho methoxyphenol ?
Question 11.16 Explain how does the –OH group attached to a carbon of benzene ring
activate it towards electrophilic substitution?
Question 11.17 Give equations of the following reactions:
(i) Oxidation of propan-1-ol with alkaline KMnO4 solution.
(ii) Bromine in CS2 with phenol.
(iii) Dilute HNO3 with phenol.
(iv) Treating phenol wih chloroform in presence of aqueous NaOH.
Question 11.18 Explain the following with an example.
(i) Kolbe’s reaction.
(ii) Reimer-Tiemann reaction.
(iii) Williamson ether synthesis.
(iv) Unsymmetrical ether.
Question 11.19 Write the mechanism of acid dehydration of ethanol to yield ethene.
Question 11.20 How are the following conversions carried out?
(i) Propene → Propan-2-ol.
(ii) Benzyl chloride → Benzyl alcohol.
(iii) Ethyl magnesium chloride → Propan-1-ol.
(iv) Methyl magnesium bromide → 2-Methylpropan-2-ol.
Question 11.21 Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid.
(ii) Oxidation of a primary alcohol to aldehyde.
(iii) Bromination of phenol to 2,4,6-tribromophenol.
(iv) Benzyl alcohol to benzoic acid.
(v) Dehydration of propan-2-ol to propene.
(vi) Butan-2-one to butan-2-ol.
Question 11.22 Give reason for the higher boiling point of ethanol in comparison to
methoxymethane.
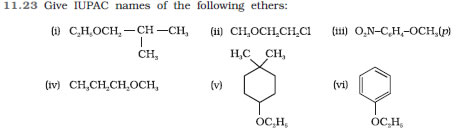
Question 11.24 Write the names of reagents and equations for the preparation of the following
ethers by Williamson’s synthesis:
(i) 1-Propoxypropane
(ii) Ethoxybenzene
(iii) 2-Methoxy-2-methylpropane
(iv) 1-Methoxyethane
Question 11.25 Illustrate with examples the limitations of Williamson synthesis for the preparation of certain types of ethers.
Question 11.26 How is 1-propoxypropane synthesised from propan-1-ol? Write mechanism of this reaction.
Question 11.27 Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Question 11.28 Write the equation of the reaction of hydrogen iodide with:
(i) 1-propoxypropane
(ii) methoxybenzene and (iii) benzyl ethyl ether.
Question 11.29 Explain the fact that in aryl alkyl ethers
(i) the alkoxy group activates the
benzene ring towards electrophilic substitution and (ii) it directs the
incoming substituents to ortho and para positions in benzene ring.
Question 11.30 Write the mechanism of the reaction of HI with methoxymethane.
Question 11.31 Write equations of the following reactions:
(i) Friedel-Crafts reaction – alkylation of anisole.
(ii) Nitration of anisole.
(iii) Bromination of anisole in ethanoic acid medium.
(iv) Friedel-Craft’s acetylation of anisole.
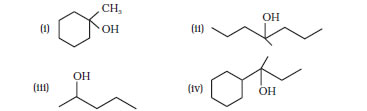
Question 11.32 Show how would you synthesise the following alcohols from appropriate
Please Wait pdf file is loading (कृपया इंतजार करें pdf file लोड हो रही है)...
Loading speed will depend up on your download speed. Pdf file के लोड होने में लगा समय आपकी डाउनलोड स्पीड पर निर्भर करेगा
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.