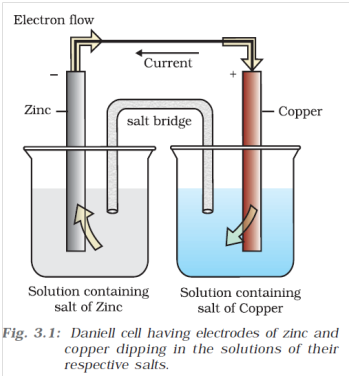
Q-3 What are the construction and functioning of Daniell cell or galvanic cell . This cell converts the chemical energy liberated during the redox reaction in to electrical energy and has an electrical potential equal to 1.1 V when concentration of Zn
2+ and Cu
2+ ions is unity (1 molar)
Zn(s) + Cu2+(aq) → Zn2+ (aq) + Cu(s)
This reaction is a combination of two half reactions whose addition gives the overall cell reaction
The reduction half reaction occurs on the copper electrode so that copper electrode may be called the reduction half cell
Cu2+ + 2e– → Cu(s)
Oxidation half reaction occurs on the zinc electrode so that zinc electrode may be called the oxidation half cell
Zn(s) → Zn2+ + 2e–
The electrolytes of the two half-cells are connected internally through a salt bridge as shown in Fig. 3.1. Sometimes, both the electrodes dip in the same electrolyte solution and in such cases we don’t require a salt bridge.
In this device the Gibbs energy of the spontaneous redox reaction is converted into electrical work which may be used for running a motor or other electrical gadgets like heater, fan, geyser, etc.
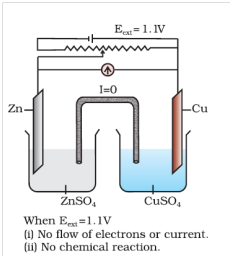
Case-1 when Ecell > External opposite potential If an external opposite potential is applied [Fig. 3.2(a)] and increased slowly, we find that the reaction continues to take place till the opposing voltage reaches the value 1.1 V [Fig. 3.2(b)]
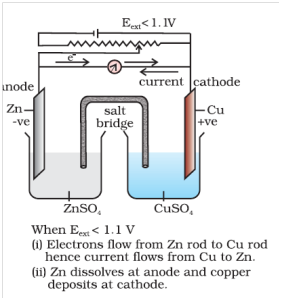 Case-2 when Ecell = External opposite potential
Case-2 when Ecell = External opposite potential When opposing voltage reaches at the value 1.1 V [Fig. 3.2(b)], the reaction stops and no further current flows through the cell takes place.
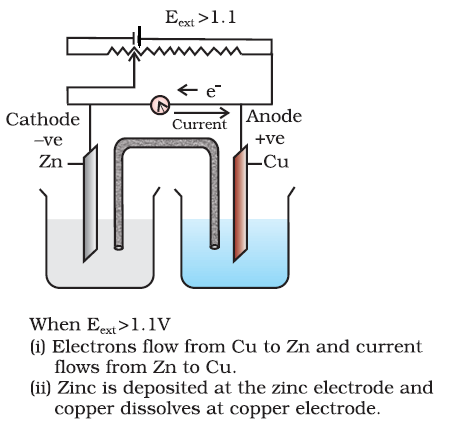
 Case- 3 when Ecell < External opposite potential
Case- 3 when Ecell < External opposite potential When opposing voltage is more than 1.1 V. The external potential again starts the reaction but in the opposite direction [Fig.3.2(c)]. It now functions as an electrolytic cell, a device for using electrical energy to carry non-spontaneous chemical reactions.