
How to measure equilibrium constant and limiting molar conductivity of week electrolytite chapter 3 can study by students of class 12. These definitiona and formulas of Class 12 Chemistry Chapter 3: Electrochemistry is developed and witten by our expert teachers. Chemistry formulas. How to measure equilibrium constant and limiting molar conductivity of week electrolytite is prepapred and collected from varius resources to help the students.
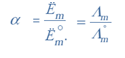
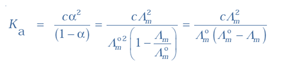
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.