
What is corrosion, how corrosion works as a cell chapter 3 can study by students of class 12. These definitiona and formulas of Class 12 Chemistry Chapter 3: Electrochemistry is developed and witten by our expert teachers. Chemistry formulas. What is corrosion explain how corrosion works as a cell is prepapred and collected from varius resources to help the students.
What is corrosion explain how corrosion works as a cell ?
Corrosion
Corrosion slowly coats the surfaces of metallic objects with oxides or other salts of the metal. The rusting of iron, tarnishing of silver, development of green coating on copper and bronze are some of the examples of corrosion.
Rusting:
At a particular spot (Fig. 3.13) of an object made of iron, oxidation takes place and that spot behaves as anode and we can write the reaction
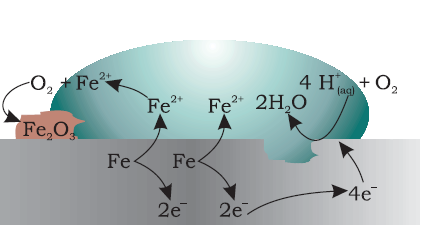
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.