
chemistry notes for class 12 chapter 9 coordination compounds download pdf, coordination compounds 12 notes, class 12 chemistry notes, coordination compounds class 12, coordination compounds class 12 notes, class 12 coordination compounds, note chemistry, chemistry notes, coordination compounds, class 12 cchemistry chapter 9 notes, 12th standard chemistry notes, 12th std chemistry notes, class 12 chemistry notes chapter 9, coordination compounds chapter class 12 notes
Coordination compounds are those addition molecular compounds which retain their identity in solid state as well as in dissolved state. In these compounds. the central metal atom or ion is linked by ions or molecules with coordinate bonds. e.g., Potassium ferrocyanide, K4 [Fe(CN)6].
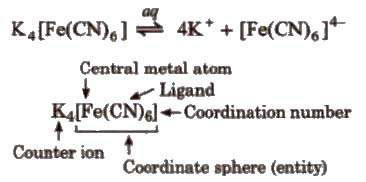
These are the addition molecular compounds which are stable in solid state but dissociate into constituent ions in the solution. e.g., Mohr’S salt, FeSO4·(NH4)2SO4 . 6H2O get dissociated into Fe2+, NH+ 4 and SO2- 4 ions.
It is an electrically charged species in which central metal atom or ion is surrounded by number of ions or neutral molecules.
It is the complex ion which carries positive charge. e.g., [Pt(NH3)4]2+
It is the complex ion which carries negative charge. e.g., Fe(CN)6]4-
The atom or ion to which a fixed number of ions or groups are bound is .ned central atom or ion. It is also referred as Lewis acid. e.g., in (NiCI2(H2O)4]. Ni is central metal atom. It is generally transition element or inner-transition element.
Ligands is electron donating species (ions or molecules) bound to the Central atom in the coordination entity.
These may be charged or neutral. LIgands are of the following types :
It is a ligand, which has one donor site, i.e., the ligand bound to a metal ion through a single donor site. e.g., H2O, NH3, etc.
It is the ligand. which have two donor sites.
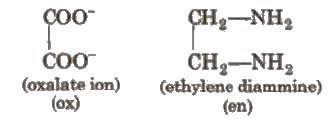
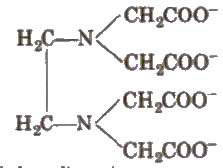
It is the ligand, which have several donor sites. e.g., [EDTA]4- is hexadentate ligand.
These are the monodentate ligands which can ligate through two different sites, e.g., NO-2, SCN-, etc.
Di or polydentate ligands cause cyclisation around the metal atom which are known as chelate IS , Such ligands USes two or more donor atoms to bind a single metal ion and are known as chelating ligands.
More the number of chelate rings, more is the stability of complex.
The stabilisation of coordination compounds due to chelation is known as chelate effect.
π – acid ligands are those ligands which can form π – bond and n-bond by accepting an appreciable amount of 1t electron density from metal atom to empty π or π – orbitals.
It is defined as the number of coordinate bonds formed by central metal atom, with the ligands.
e.g., in [PtCI6]2-, Pt has coordination number 6.
In case of monodentate ligands,
Coordination number = number of ligands
In polydentate ligands.
Coordination number = number of ligands * denticity
The central ion and the ligands attached to it are enclosed in square bracket which is known as coordination sphere. The ionisable group written outside the bracket is known as counter ions.
The spatial arrangement of the ligands which are directly attached to the central atom or ion, is called coordination polyhedron around the central atom or ion.
The charge of the complex if all the ligands are removed along with the electron pairs that are shared with the central atom, is called oxidation number of central atom.
e.g., [CU(CN4)3-, oxidation number of copper is +1, and represented as Cu(I).
Complexes in which the metal atom or ion is linked to only one kind of donor atoms, are called homoleptic complexes e.g., [Co(NH3)6]3+
Complexes in which the metal atom or ion is linked to more than one kind of donor atoms are called heteroleptic complexes e.g., [Co(NH3)4CI2]+
Complexes in which the ligand substitution is fast are known as labile complexes and in which ligand substitution is slow, are known as inert complexes.
This concept was proposed by Sidgwick. In a complex, the EAN of metal atom is equal to the total number of electrons present in it.
EAN = Z – ON of metal + 2 * CN
(where, Z = atomic number of metal atom
ON = oxidation number of metal
and CN = coordination number of complex)
An ion with central metal atom having EAN equal to next inert gas will be more stable.
Naming is based on set of rules given by IUPAC.
1. Name of the compound is written in two parts (i) name of cation, and (ii) name of anion.
2. The cation is named first in both positively and negatively charged coordination complexes.
3. The dissimilar ligands are named in au alphabetical order before the name of central metal atom or ion.
4. For more then one similar ligands. the prefixes di, tri, tetra, etc are added before its name. If the di, tri, etc already appear in the complex then bis, tris, tetrakis are used.
5. If the complex part is anion, the name of the central metal ends with suffix ‘ate’.
6. Names of the anionic ligands end in ‘0’, names of positive ligands end with ‘ium’ and names of neutral ligands remains as such. But exception are there as we use aqua for H2O, ammine for NH3, carbonyl for CO and nitrosyl for NO.
7. Oxidation state for the metal in cation, anion or neutral coordination compounds is indicated by Roman numeral in parentheses.
8. The name of the complex part is written as one word.
9. If the complex ion is a cation, the metal is named same as the element.
10. The neutral complex molecule is named similar to that of the complex cation.
Some examples are
(i) [Cr(NH3)3(H2O)3]Cl3
triamminetrichlorochromium (III) chloride
(ii) [Co(H2CH2CH2H2)3]2(SO4)3
tris (ethane-l,2-diamine) cobalt (III) sulphate
(iii) [Ag(NH 3)2 ] [Ag(CN)2]
diamminesilver (I) dicyanoargentate(I)
(iv) K4 [Fe(CN)6]
potassium hexacyanoferrate (II)
Coordination compounds exhibit the following types of isomerism:
In this isomerism. isomers have different bonding pattern. Different types of structural isomers are
This type of isomerism i s shown by the coordination compounds having ambidentate ligands. e.g.,
[Co(NH3)5(NO2)]Cl and [Co(NH3)5(ONO)]Cl or pentaammine nitrito- N Cobalt (III) chloride and pentaammine nitrito-O’Cobalt (III) chloride.
This type of isomerism arises from the interchange of ligands between cationic and anionic complexes of different metal ions present in a complex, e.g.,
[Cr(NH3)6) [CO(CN)6] and [CO(NH3)6] [Cr(CN)6]
This isomerism arise due to exchange of ionisable anion with anionic ligand. e.g..
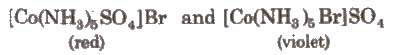
This is also known as hydrate isomerism. In this isomerism, water is taken as solvent. It has different number of water molecules in the coordination sphere and outside it. e.g..
[Co(H2O)6]CI3, [Co(H2O)4C12]Cl·2H2O, [Co(H2O)3Cl3]. 3H2O
Stereoisomers have the same chemical formula and chemical bonds but they have different spatial arrangement. These are of two types :
Geometrical isomers are of two types i.e., cis and trans isomers. This isomensm is common in complexes with coordination number 4 and 6.
(i) Tetrahedral complexes do not show geometrical isomerism.
(ii) Square planar complexes of formula [MX2L2] (X and L are unidentate) show geometrical isomerism. The two X ligands may be arranged adjacent to each other in a cis isomer, or opposite to each other in a trans isomer, e.g.,
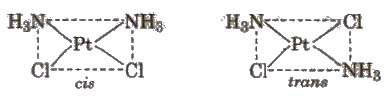
(iii) Square planar complex of the type [MABXL] (where A, B, X, L, are unidentate ligands) shows three isomers, two cis and one trans.
e.g., [Pt(NH3) (Br)(Cl)(Py)].
Octahedral complexes of formula [MX2L4], in which the two X ligands may be oriented cis or trans to each other, e.g., [Co(NH3)4Cl2)+.
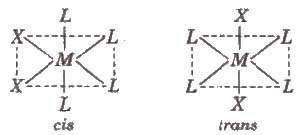
Octahedral complexes of formula [MX2A2], where X are unidentate ligands and A are bidentate ligand. form cis and trans isomers, e.g., [CoC12(en)2]’
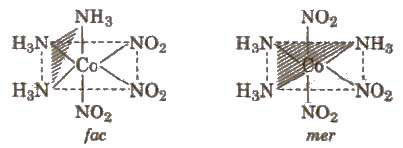
In octahedral complexes of formula [MA3X3], if three donor atoms of the same ligands occupy adjacent positions at the corners of an octahedral face. it is known as facial (fae) isomer, when the positions are around the meridian of the octahedron, it is known as meridional (mer) isomer. e.g., [Co(NH3)3 (NO2)3]
(ii) Optical isomerism These are the complexes which have chiral structures. It arises when mirror images cannot be superimposed on one another. These mirror images are called enantiomers. The two forms are called dextro (d) and laevo (l) forms.
Tetrahedral complexes with formula [M(AB)2] show optical isomers and octahedral complexes (cis form) exhibit optical isomerism.
Metals exhibit two types of valencies in the formation of complexes.
These are primary valencies and secondary valencies.
1. Primary valencies correspond to oxidation number (ON) of the metal and are satisfied by anions. These are ionisable and non-directional.
2. Secondary valencies correspond to coordination number (CN) of the metal atom and are satisfied by ligands. These are non-ionisable and directional. Hence, geometry is decided by these valencies.
This theory was proposed by L. Pauling in 1930 s. According to this theory, when a complex is formed, the metal ion/atom provides empty orbitals to the surrounding ligands. Coordination number shows the number of such empty orbitals, i.e., number of empty orbitals is equal to the coordination number. These empty orbitals hybridised before participation in bonding and the nature of hybridisation depends on the nature of metal and on the nature of approaching ligand.
When outer d-orbital are used in bonding, the complexes are called outer orbital complexes. They are formed due to weak field ligands or high spin ligands and hybridisation is sp3d2. They have octahedral shape.
When d-orbitals of (n – 1) shell are used, these are known as inner orbital complex, they are formed due to strong field ligands or low spin ligands and hybridisation is d2sp3. They are also octahedral in shape.
1. 6 – ligands (unidentate), octahedral entity.
(i) Inner orbital complex [Co(NH3)6]3+
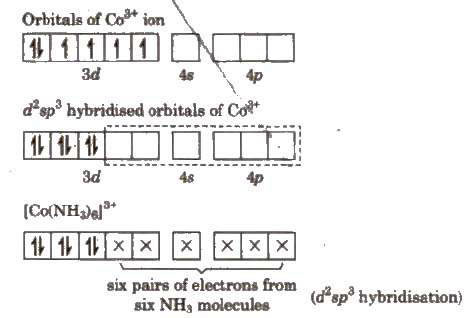
All electrons are paired, therefore complex will be diamagnetic in nature.
(ii) Outer orbital complex, [CoF6]3-
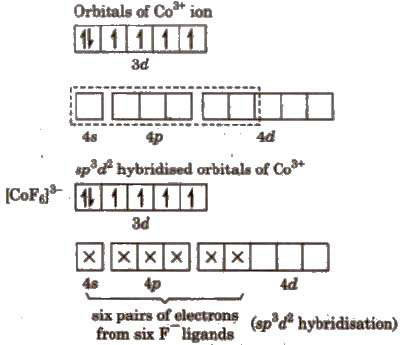
Complex has unpaired electrons, therefore, it will be paramagnetic in nature.
2. 4-ligands (unidentate) tetrahedral entity.

(i) Inner orbital complex, [Ni(CN)4]2-
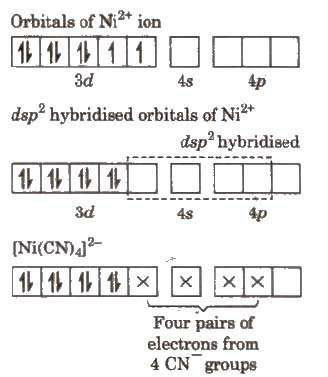
All electrons are paired so complex will be diamagnetic in nature.
(ii) Outer orbital complex, [CoCI4]-
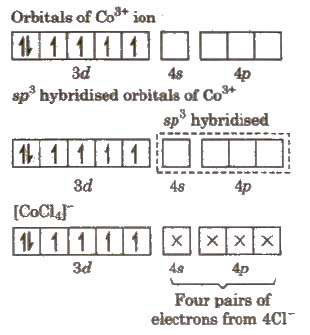
Since, complex has unpaired electrons. so it will be paramagnetic in nature.
This theory could not explain the quantisation of the magnetic data, existence of inner orbita and outer orbital complex, change of magnetic moment with temperature and colour of complexes.
This theory was proposed by H. Bethe and van Vleck. Orgel. in 1952, applied this theory to coordination compounds. In this theory, ligands are treated as point charges in case of anions and dipoles in case of neutral molecules.
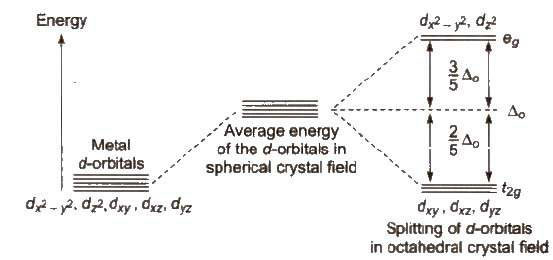
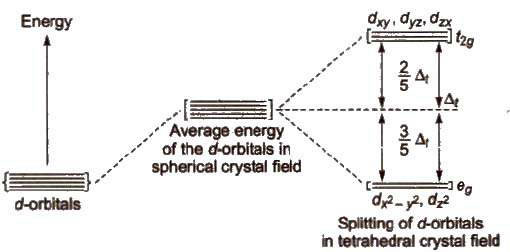
The five d-orbitals are classified as
(i) Three d-orbitals i.e., dxy, dyz and dzx are oriented in between the coordinate axes and are called t2g – orbitals.
(ii) The other two d-orbitals, i.e., d x 2 - y 2 and d z 2 oriented along the x – y % axes are called eg – orbitals.
Due to approach of ligands, the five degenerate d-orbitals split. Splitting of d-orbitals depends on the nature of the crystal field.
[The energy difference between t2g and eg level is designated by Δ and is called crystal field
splitting energy.]
By using spectroscopic data for a number of coordination compounds, having the same metal ions but different ligand, the crystal field splitting for each ligand has been calculated. A series in which ligand are arranged in order of increasing magnitude of crystal field splitting, is called spectrochemical series.
Spectrochemical series
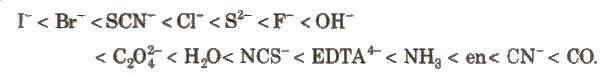
In case of octahedral complexes, energy separation is denoted by Δo (where subscript 0 is for
octahedral).
In octahedral complexes, the six-ligands approach the central metal ion along the axis of d x
2 -
y
2 and d z
2 orbitals.
Energy of eg set of orbitals > energy of t2g set of orbitals.
The energy of eg orbitals will increase by (3/5) Δo and t2g will decrease by (2/5) Δo.
If Δo < P, the fourth electron enters one of the eg orbitals giving the configuration t3
2g e1
g.
Ligands for which Δo < P are known as weak field ligands and form high spin complexes.
If Δo > P, it becomes more energetically favourable for the fourth electron to occupy a
t2g orbital with configuration t4
2g eo
g. (where, P = energy required for e- pairing in an orbital).
Ligands which produce this effect are known as strong field ligands and form low spin
complexes.
In tetrahedral complexes, four ligands may be imagined to occupy the alternate comers of the cube and the metal ion at the center of the cube.
Energy of t2g set of orbitals > Energy of eg set of orbitals.
In such complexes d – orbital splitting is inverted and is smaller as compared to the octahedral field splitting.
Orbital splitting energies are so low that pairing of electrons are not possible so these are high spin complexes.
The crystal field theory attributes the colour of the coordination compounds to dod transition of the electron, i.e., electron jump from t2g level to higher eg level.
In the absence of ligands, crystal field splitting does not occur and hence the substance is colourless.
1. It does not consider the formation of 7t bonding in complexes.
2. It is also unable to account satisfactorily for the relative strengths of ligands e.g., it does not
explain why H2O is stronger ligand than OH-.
3. It gives no account of the partly covalent nature of metal-metal bonds.
This theory was put forward by Hund and Mulliken. According to this theory, all the atomic orbitals of the atom participating in molecule formation get mixed to give rise an equivalent number of new orbitals, called the molecular orbitals. The electrons are now under the influence of all the nuclei.
The stability of complex in solution refers to the degree of association between the two species involved in the state of equilibrium. It is expressed as stability constant (K).
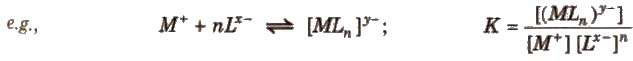
The factors on which stability of the complex depends :
(i) Charge on the central metal atom As the magnitude of charge on metal atom increases,
stability of the complex increases.
(ii) Nature of metal ion The stability order is 3d < 4d < 5d series.
(iii) Basic nature of ligands Strong field ligands form stable complex.
The instability constant or the dissociation constant of compounds is defined as the reciprocal of the formation or stability Constant.
1. They are used in many qualitative and quantitative analysis.
2. Hardness of water is estimated by simple titration with Na2 EDTA.
3. Purification of metals can be achieved through formation and subsequent decomposition of
their coordination compounds.
4. They have great importance in biological systems.
5. They are used as catalyst for many industrial processes.
6. In medicinal chemistry, there is a growing interest of chelating therapy.
They contain one or more metal-carbon bond in their molecules. They are of the following types:
Metal-carbon bond is sigma bond, e.g., (C2H5)4 Pb, Zn(C2H5)2 R – Mg – X, etc.
In which molecules/ions containing π bonds act as a ligand. e.g., Ferrocene, Dibenzene chromium and Zeise’s salt.
Zeise’s salts is K[PtCI3(η2 – C2H4)] In which ethylene acts as a ligand which do not have a lone pair oi electron.
In ferrocene, Fe(η5 – C5H5)2 represents the number of carbon atoms with which metal ion is directly attached.
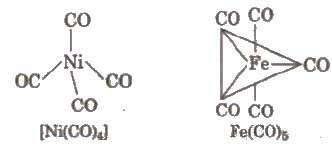
Metal carbonyls are their examples. Metal-carbon bond of metal carbonyls have both σ and π – bond character. They have CO molecule as ligand, e.g.,
Wilkinson’s catalyst (Rh(PPh3)3CI] is used as homogeneous catalyst in the hydrogenation of alkenes. Zeigler-Natta catalyst
[Ti CI4 + (C2H5>3Al] acts as heterogeneous catalyst in the polymerisation of ethylene
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.