
chemistry class 12 notes, chemistry notes for class 12 pdf, chemistry notes for class 12, class 12 chemistry notes, notes of chemistry class 12, chemistry notes class 12, organic chemistry notes for class 12, chemistry notes for class 12 free download, chemistry notes for class 12 free download pdf, chemistry notes for class 10, chemistry 12 notes, class 10 chemistry notes, 12 chemistry notes, chemistry in everyday life class 12 notes, basic chemistry notes, igcse chemistry notes , surface chemistry 12 notes, class 12 chemistry notes, surface chemistry class 12, surface chemistry class 12 notes, class 12 surface chemistry, note chemistry, chemistry notes, surface chemistry, class 12 cchemistry chapter 5 notes, 12th standard chemistry notes, 12th std chemistry notes, class 12
The accumulation of molecular species at the surface rather than in the bulk of a solid or liquid
The molecular species or substance, which concentrate at the surface. Adsorbent:The material on the surface of which the adsorption takes place. Adsorption is essentially a surface phenomenon.
The process of removing an adsorbed substance from a surface on which it is adsorbed.
Factors featuring adsorption
The extent of adsorption increases with the increase of surface area per unit mass of the adsorbent at a give temperature and pressure. Easily liquefiable gases(i.e., with higher critical temperatures) are readily asodrbed Adsorption is accompanied by decrease in enthalpy as well as decrease in entropy of the system.
Physical adsorption |
Chemical adsorption |
1.It arises because of van der Waals‘ forces. 2.It is not specific in nature. 3. It is reversible in nature. 4. Enthalpy of adsorption is low (20-40 kJ mol–1 )in this case 5. No appreciable activationenergy is needed. 6. It results into multimolecular layers on adsorbent surface under high pressure |
1.It is caused by chemical bond formation. 2. It is highly specific in nature. 3. It is irreversible. 4. Enthalpy of adsorption is high (80-240 kJ mol–1) in this case 5.High activation energy is sometimes needed. 6.It results into unimolecular layer |
Empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature. x/m= k.P1/n (n > 1)
where x is the mass of the gas adsorbed on mass m of the adsorbent at pressure P, k and n are constants which depend on the nature of the adsorbent and the gas at a particular temperature.
T
aking logarithm
log x/m= log k + 1/n log P
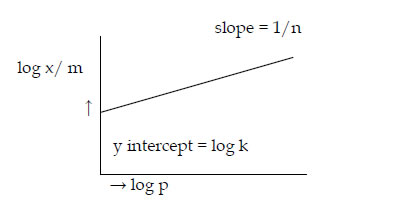
When 1/n = 0, x/m = constant, the adsorption is independent of pressure. When 1/n = 1, x/m= k P, the adsorption varies directly with pressure.
Catalysts - Substances, which alter the rate of a chemical reaction andthemselves remain chemically and quantitatively unchanged after the reaction.
Promoters - Substances that enhance the activity of a catalyst.
For example, in Haber‘s process for manufacture of ammonia, molybdenum acts as a promoter for iron which is used as a catalyst.
Poisons – Substances that decrease the activity of a catalyst.
When the reactants and the catalyst are in the same phase (i.e.,liquid or gas.
Eg:- Oxidation of sulphur dioxide into sulphur trioxide with dioxygen in the presence of oxides of nitrogen as the catalyst in the lead chamber process.
![]()
The catalytic process in which the reactants and the catalyst are in different phases. is known as heterogeneous catalysis.
Eg:- Oxidation of sulphur dioxide into sulphur trioxide in the presence of Pt.
2SO2.(g)→ 2SO3(g)
The mechanism of heterogeneous catalysis involves five steps:
(i) Diffusion of reactants to the surface of the catalyst.
(ii) Adsorption of reactant molecules on the surface of the catalyst.
(iii) Occurrence of chemical reaction on the catalyst‘s surface through formation of an intermediate
(iv) Desorption of reaction products from the catalyst surface.
(v) Diffusion of reaction products away from the catalyst‘s surface.
The activity of a catalyst depends upon the strength of chemisorptions to a large extent. The reactants must get adsorbed reasonably strongly on to the catalyst (but not so strongly) to become active. Eg:- 2H2 (g) + O2 (g) Pt→ 2 H2O(l)
The selectivity of a catalyst is its ability to direct a reaction to yield a particular product.
Eg:-
Shape-selective catalysis: The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant. Zeolites are good shape-selective catalysts.
Eg:- ZSM-5 converts alcohols directly into gasoline (petrol) by dehydrating them to give a mixture of hydrocarbons.
Enzymes :The enzymes are biochemical catalysts.
Eg:- Inversion of cane sugar: The invertase enzyme converts cane sugar into glucose and fructose.
A colloid is a heterogeneous system in which one substance is dispersed (dispersed phase) as very fine particles in another substance called dispersion medium. Range of diameters is between 1 and 1000 nm.
(i)Based on PhysicalState of Dispersed Phase and Dispersion Medium See table 5.4 NCERT text book
Solids in liquids – sols
eg:- starch sol
Liquids in solids – gels eg:- butt er
Liquids in liquids – emulsions eg:- milk
(ii)Based on nature of interaction between dispersed phase and the dispersion medium
Lyophilic colloids |
Lyophobic colloids |
| 1.Solvent liking 2.Reversible sols 3.Quite stable 4.Cannot be easily Coagulated |
1. Solvent hating 2.Irreversible sols 3.Unstable.Need stabilising agents to preserve 4.Can be coagulated easily by adding small amount of electrolyte |
(iii)Based on the type of the particles of the dispersed phase
Multimolecular colloids |
Macromolecular colloids |
Associated colloids (Micelles |
| Atoms or molecules aggregate together to form colloidal range species .Eg:- gold sol,sulphur sol |
Solutions in which the size of the macro molecules may be in the colloidal range. Eg:- starch sol |
At low concentrations behave as normal strong electrolytes, but at higher concentrations exhibit colloidal behaviour due to the formation of aggregates Eg:- soaps & detergents |
Temperature above which the formation of micelles takes place.
Concentration above which the formation of micelles takes place.
Peptization_ Process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte.
It is a process of removing a dissolved substance from a colloidal solution by means of diffusion through a suitable membrane.
Dialysis can be made fasterby applying an electric field if the dissolved substance in the impure colloidal solution is only an electrolyte.. idal solution.
The scattering of light rays by colloidal particles due to which the path of light is illuminated. Tyndalleffect is observed only when
(a) The diameter of the dispesed particles is not much smaller than the wave length of the light used
(b) The refractive indices of the dispersed phase and the dispersion medium differ greatly in magnitude
The zig-zag movement of colloidal particles due to the unbalanced bombardment of the dispersed particles with the molecules of the dispersion medium.
The movement of colloidal particles under an applied electric potential Charge on colloidal particles :- The colloidal particles develop charge due to the following reasons
(a) Electron capture by sol particles during electrodispersion of metals
(b) Due to preferential adsorption of ions from solutions
(c) Due to formulation of electrical double lay Coagulation or precipitation of colloidal
the process of settling of colloidal particles. Caused due to
(a) addition of electrolytes
(b) electrophoresis
(c) boiling (d)mixing two oppositely charged sols
Greater the valence of the coagulating ion added to a sol, the greater is its power to cause precipitation . The coagulation power of some of the cations Al3+> Ba2+ >Na+. The coagulating power of some of the anions is in the order [Fe(CN) 6] 4- >PO43->SO 42->Cl-
Coagulating value of an electrolyte: The minimum concentration of an electrolyte in millimols per litre required to cause precipitation of a sol in two hours.
colloidal system where a liquid is dispersed in another liquid.
Types of emulsions
(a) oil in water type (o/w) eg: milk, vanishing cream
(b) water in oil type (w/o) eg: butter , cold cream
Emulsions are stabilized by emulsifying agents . eg : soaps
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.