
Reaction of metals with oxygen, Reaction of metals with water, Reaction of metals with Aci chapter 3 can study by students of class 10. These definitiona and formulas of Class 10 Science Chapter 3: Metal and Non-metal is developed and witten by our expert teachers. Science formulas. Reaction of metals with oxygen, Reaction of metals with water, Reaction of metals with Acids, Difference between Metals and Non-metals is prepapred and collected from varius resources to help the students.
Reaction of metals with oxygen: Generally all metals combine with oxygen to form metal oxide if we supply some heat.
Reaction of metals with water: Most of the metals react with water to form metal hydroxide and release hydrogen gas. For example just see the following chemical equation.
Reaction of metals with Acids: When metals react with acids they generally displace hydrogen from dilute acids.
Difference between Metals and Non-metals on the basis of their physical properties:
Metals | Non-metals |
1. Metals are generally solid at room temperature. (Except Mercury) | 1. Non-metals exist in all the three states i.e solid liquid and gas. |
2. Metals are generally hard. (except sodium which can be cut using a knife) | 2. Non-metals are generally soft. (except diamond which us the hardest substance on the earth) |
3. Metals are Malleable; means can be converted into thin sheets using a hammer. (except sodium and potassium) | 3. Non-metals are generally brittle. Will broke down into pieces when beaten by a hammer. |
4. Metals are ductile, means they can be converted into thin wires. | 4. Non-metals are non ductile. |
5. Metals have shiny surface or they are lustrous. | 5. They are non lustrous. (except iodine) |
6. Metals can lose electrons so they are electropositive in nature. | 6. Non-metals gain electrons so they are electronegative in nature. |
7. Metals are good conductors of heat and electricity. | 7. Non-metals are bed conductors of heat and electricity. (Except graphite) |
8. Metals are sonorous. They produce a sound on striking with a hard surface. | 8. They does not do so they are not sonorous. |
9. Metals generally have high densities. (except alkali metals) | 9. Non-metals generally have low densities. |
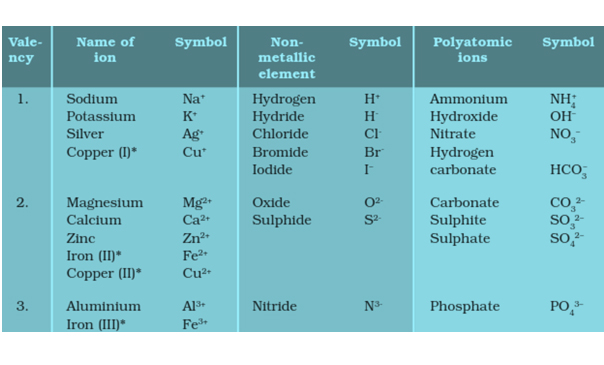
Some common, simple and Polyatomic Ions: Must learn to make chemical formula of a compound.
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.