
What is Matter, Classification of Matter, Properties of Matter chapter 1 can study by students of class 11. These definitiona and formulas of Class 11 Chemistry Chapter 1: Some Basic Concepts of Chemistry is developed and witten by our expert teachers. Chemistry formulas. What is Matter, Classification of Matter and it’s properties is prepapred and collected from varius resources to help the students.
What is matter ?
Anything which has mass and occupies space is called matter. for example, book, pen, pencil, water, air, all living beings etc. are composed of matter.
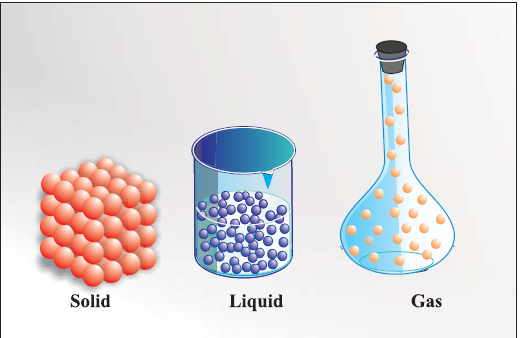
matter can exist in three physical states viz. solid, liquid and gas.
Solid :
Solids have definite volume and definite shape.
Reason In solids, these particles are held very close to each other in an orderly fashion and there is not much freedom of movement.
Liquid
Liquids have definite volume but not the definite shape. They take the shape of the container in which they are placed.
Reason In liquids, the particles are close to each other but they can move around
Gas:
Gases have neither definite volume nor definite shape. They completely occupy the container in which they are placed.
Reason :in gases, the particles are far apart as compared to those present in solid or liquid states and their movement is easy and fast
Classification of matter:
Matter can be classified as mixtures or pure substances.
Mixture : A mixture contains two or more substances present in it (in any ratio) which are called its components.
For example, sugar solution in water, air, tea etc.
Mixtures are two types
heterogeneous mixtures : the components completely mix with each other and its composition is uniform throughout.
Examples Sugar solution, air
heterogeneous mixtures: the composition is not uniform throughout and sometimes the different components can be observed.
Example: mixtures of salt and sugar
What are the method of separation of component of mixture?
components of a mixture can be separated using physical methods such as simple hand picking, filtration, crystallisation, distillation etc
Pure substances. They have fixed composition . constituents components of pure substances cannot be separated by simple physical methods.
Examples Copper, silver, gold, water, glucose etc
Pure substances can be further classified into elements and compounds
Elements: An element consists of only one type of particles. These particles may be atoms or molecules.
Examples: Sodium, copper, silver, hydrogen, oxygen etc.
Compound: When two or more atoms of different elements combine, the molecule of a compound is obtained. the atoms of different elements are present in a compound in a fixed and definite ratio and this ratio is characteristic of a particular compound. the properties of a compound are different from those of its constituent elements. Constituent particles can be separated by chemical methods.
The examples of some compounds are water, ammonia, carbon dioxide, sugar etc
What are the physical properties of the matter?
Physical properties are those properties which can be measured or observed without changing the identity or the composition of the substance.
Examples: colour, odour, melting point,boiling point, density etc
What are the chemical properties?
Chemical properties are those properties which require a chemical changes to occur
Examples: acidity or basicity, combustibility etc.
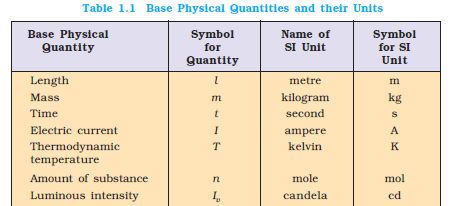
1 mol : The mole is the amount of substance of a system which contains as many elementary
entities as there are atoms in 0.012 kilogram of carbon-12; its symbol is “mol”.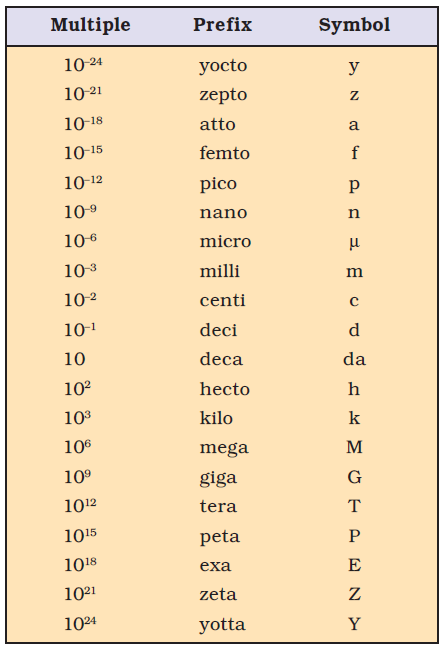
Pico ,nano, micro , mili ,deci,centi ,deca, hecta, kilo and mega is important for exams and numerical problems
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.