
metals and nonmetals class 10, metals and nonmetals class 10 notes, metals and nonmetals class 8, properties of metals and nonmetals, about metals and nonmetals, metal and nonmetals, what are metals and nonmetals, , metal and non-metal 10 notes, class 10 science notes, metal and non-metal class 10, metal and non-metal class 10 notes, class 10 metal and non-metal, note science, science notes, metal and non-metal, class 10 cscience chapter 3 notes, 10th standard science notes, 10th std science notes, class 10 science notes chapter 3, metal and non-metal chapter class 10 notes
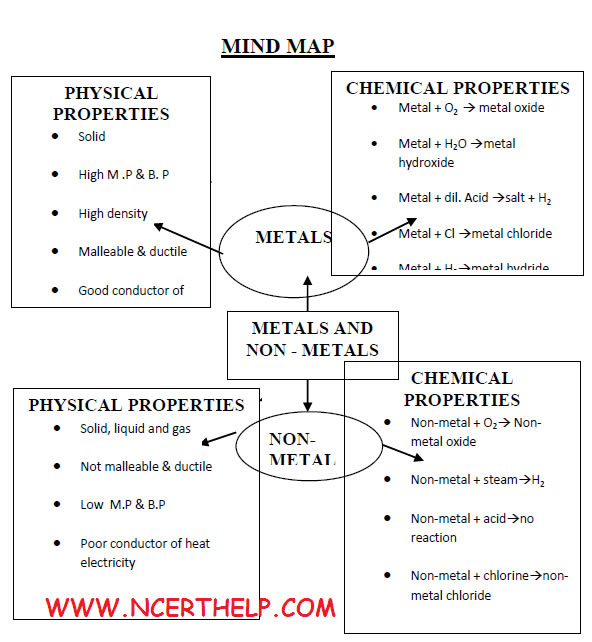
Elements are classified broadly into two categories on the basis of properties: Metals: Iron, Zinc, Copper, Aluminium etc.
Non – metals: Chlorine, Nitrogen, Hydrogen, Oxygen, Sulphur etc.Apart from metals and non-metals some elements show properties of both metals and non – metals, e.g. Silicon, Arsenic, Germanium .They are called metalloids
S.No |
Property |
Metals |
Non-Metals |
1 |
Physical State |
Metals are solid at room |
Non-metals generally |
2 |
Melting and boiling points |
Metals generally have |
Non-metals have low |
3 |
Density |
Generally high. |
Generally low. |
4 |
Malleability and Ductility |
Malleable and ductile. |
Neither malleable nor |
5 |
Electrical and thermal conductivity |
Good conductors of heat |
Generally poor |
6 |
Luster |
Poses shining luster. |
Do not have luster |
7 |
Sonorous sound |
Give sonorous sound |
Does not give |
8 |
Hardness |
Generally hard except |
Solid non-metals are |
1 |
Reaction
with |
Metal + Oxygen→Metal oxide |
Non-metal + Oxygen →Non-metal oxide |
2 |
Reaction |
Metals react with water to |
Non-metals do not react |
3 |
Reaction |
Metal + Acid →Metal salt
+ Hydrogen HCl |
Non-metals do not react
with acids to release H2 gas |
4 |
Reaction |
When metals react with salt |
When non-metals react |
5 |
Reaction with Chlorine |
Metal + Chlorine→ Metal Chloride |
Non-metal + Chlorine→Non-metal Chloride covalent bond is formed. Therefore covalent compound is obtained. H2(g) + Cl2 → 2HCl
|
| 6 | Reaction |
Metals react with hydrogen |
Non-metals react with hydrogen to form hydrides H2(g) + S(l) → H2S(g) |
Properties of ionic compounds |
||||
| 1. | Physical nature |
: | solid and hard due to strong force of attraction. (generally brittle) | |
| 2. | Melting point and boiling point |
: | have high M.P and B.P, as large amount of heat energy is required to break strong ionic attraction. | |
| 3. | Solubility |
: | soluble in water and insoluble in kerosene and pertrol. | |
| 4. | Conduction of electricity |
: | ionic compounds in solid state-----does not conduct electricity. | |
Reason—Ions can not move due to rigid solid structure. Ionic compounds conduct electricity in molten state. |
||||
Reason-- Ions can move freely since the electrostatic forces of attraction between the oppositely charged ions are overcome due to heat. |
||||
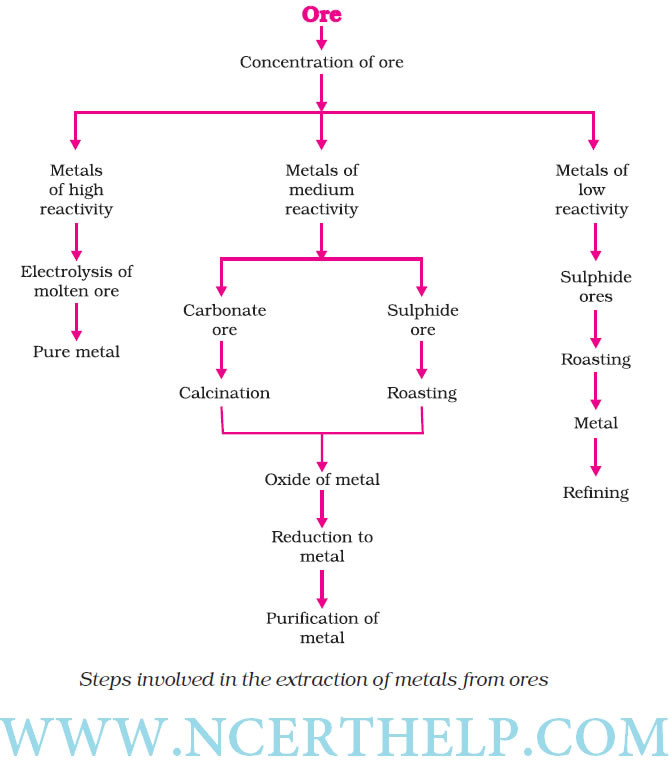
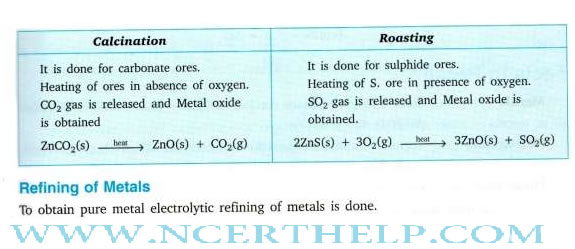
Occurrence of metals. It occurs in Earths crust, sea-water
|
||||
Minerals Elements or compounds, occuring naturally in the earth‘s crust |
Ores Minerals that contain very high percentage of a perticular metal and these met als can be extracted economically on a large scale. |
|||
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.