
Ncert Solutions for class 9 subject Science Chapter 1 Matter In Our Surroundingin pdf Best Free NCERT Solutions for class 1 to 12 in pdf NCERT Solutions, cbse board, Science, ncert Solutions for Class 9 Science, class 9 Science ncert solutions, Matter In Our Surrounding, Class 9, ncert solutions chapter 1 Matter In Our Surrounding, class 9 Science, class 9 Science ncert solutions, Science ncert solutions class 9, Ncert Solutions Class 9 Science Chapter 1 Matter In Our Surrounding
Matter: Any substance which occupies space and has mass is known as matter. Early Indian philosophers classified matter in five basic elements: air, earth, water, fire and sky that also known as Panch Tatva.
States of matter: Matter exist in three different states that are Solid, Liquid and Gas.
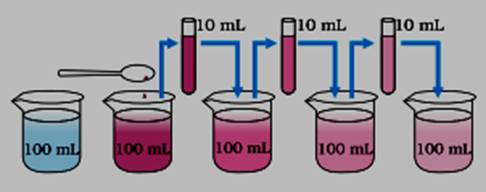
Matter is made up of very small particles: All matter is made up of very small particles that are not visible to naked eye. We can prove this by performing the following experiment: Take two or three crystals of potassium permanganate and add in 100ml of water. We will see that the colour of the water gets changed. Now take 10ml of this solution and add it into another 100ml of fresh water, again you will observe that the colour of water will change but it is not that much dark as in first case. Now repeat this procedure three more time still you will find that the colour of the water gets change but it will become fainter and fainter.
Characteristics of particles of matter: The characteristics of particles of matter are
(i) Particles of matter have spaces between them.
(ii) Particles of matter are continuously moving.
(iii) Particles of matter attract each other.
(iv) Kinetic energy of particles increases with increase in temperature.
Properties of solids: Properties of solids
(i) Solids have definite shape and distinct boundaries.Properties of liquids: Properties of liquids
(i) Liquids do not have definite shape and distinct boundaries.
(ii) Liquids have fixed volume.
(iii) They can be compressed.
(iv) They take up the shape of the container (they can change its shape).
(v) They are fluid and thus can flow like water.
(vi) Their intermolecular force of attraction is less than solids.
(vii)The kinetic energy of its particles is more than solids.
Properties of gas: Properties of gas
(i) Gases neither have definite shape nor have fixed volume.Effect of change of temperature on matter: On increasing the temperature of solids, the kinetic energy of the particles increases.
Due to the increase in kinetic energy, the particles start vibrating with greater speed. The energy supplied by heat overcomes the forces of attraction between the particles. The particles leave their fixed positions and start moving more freely. A stage is reached when the solid melts and is converted to a liquid. The temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
Effect of change of pressure on matter: By applying pressure we can bring the particles of matter closer and closer. So by applying pressure solids can be converted into liquids and further applying more pressure liquids can be converted into gases.
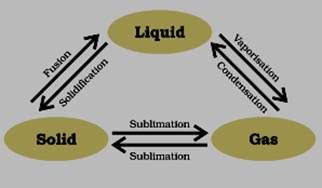
Interconversion of three states of matter: Any state of matter can be converted into other state with the help of temperature or pressure. Different terminology for the Interconversion of states of matter is given below:
(i) Solid to Liquid: FusionObserve the following diagram to memorise this Interconversion states of matter:

Evaporation: The phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation. For example spreaded water on the floor disappears after some time even when the temperature of the surface of the floor is much lower than the boiling point of water.
What are the various factors that affect evaporation?
Various factors that affect evaporation are:
How does evaporation cause cooling?
When a liquid keeps on evaporating. The particles of liquid absorb energy from the surrounding to regain the energy lost during evaporation. This absorption of energy from the surroundings makes the surroundings cold.
Melting Point: The temperature at which the solid starts melting to become a liquid at the atmospheric pressure is called its melting point.
Boiling Point: The temperature at which the liquid starts boiling at the atmospheric pressure is called its boiling point.
Latent heat of fusion: The amount of heat energy required to change 1 kg of solid into liquid at atmospheric pressure at its melting point is called the latent heat of fusion.
Latent heat of vaporisation: The amount of heat energy required to change 1 kg of liquid into gas at atmospheric pressure at its boiling point is called the latent heat of vaporisation.
Sublimation: There are some substances that change directly from solid state to gaseous state and vice versa without changing into liquid state. Such substances are called sublime and this process in known as sublimation.
Why do we see water droplets on the outer surface of the glass containing ice-cold water?
Sometimes we see water droplets on the outer surface of the glass containing ice-cold water this is because the water vapour presents in air, on coming in contact with the cold glass of water, loses energy and gets converted to liquid state, which we see as water droplets.
What is Dry Ice?
We know that gases can be liquefied by applying pressure and reducing temperature. When carbon dioxide is stored under very high pressure it becomes solid and known as solid CO2. Solid CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without coming into liquid state. This is the reason that solid carbon dioxide is also known as dry ice.
In text questions page number 3
Question 1. Which of the following is matter?
Chair, air, love, smell, hate, almonds, thought, cold, cold drink, smell of perfume.
Answer: Chair, air, almonds and cold drink are matter.
Question 2. Give reasons for the following observation:
The smell of hot sizzling food reaches you several metres away, but to get the smell from cold food you have to go close.
Answer: The smell of hot sizzling food reaches you several metres away, but to get the smell from cold food you have to go close because the kinetic energy of the particles of matter increases with the increase in temperature.
Question 3. A er is able to cut through water in a swimming pool. Which property of matter does this observation show?
Answer: A er is able to cut through water in a swimming pool this observation shows that the matter is made up of particles.
Question 4. What are the characteristics of the particles of matter?
Answer: The characteristics of particles of matter are
In text questions page number 6
Question 1. The mass per unit volume of a substance is called density. (Density = mass/volume).
Arrange the following in order of increasing density – air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Answer: Arrangement of the above matters in order of increasing density
Air<exhaust from chimney<cotton<water<honey<chalk<Iron
Question 2. (a) Tabulate the differences in the characteristics of states of matter.
(b) Comment upon the following:
rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Answer: (a)
| S.NO | Property | Solid | Liquid | Gases |
1. | Shape | They Have definiteshape | They do not havedefinite shape | They do not havedefinite shape |
2. | Volume | They have definitevolume | They do not havedefinite volume | They do not havedefinite volume |
3. | Density | They have highdensity. | Liquids have lessdensity than solids. | Liquids have leastdensity. |
4. | Kinetic energy ofparticles. | Least | More than solids | Maximum |
5. | Compressibility | Negligible | Low | High |
Question 3. Give reasons
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
Answer:
Quesiton 4. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Answer: Ice in fact it is solid but it has cage like structure and hence its molecules have more empty space as compared to water so it has smaller density then water and hence floats on water.
In text questions page number 9
Question1. Convert the following temperature to Celsius scale: a. 300 K b. 573 K.
Answer: To concert Kelvin scale into Celsius scale we have to subtract 273 so (a) 300K=300-273=27 ºC, (b) 573K=573-273=300 ºC
Question 2. What is the physical state of water at: a. 250ºC, b. 100ºC?
Answer: (a) At 250ºC water exist in only gaseous state. (b) At 100ºC water exist in both gaseous and liquid state.
Quesiton 3. For any substance, why does the temperature remain constant during the change of state?
Answer: The temperature remain constant during the change of state because the heat supplied is used up in changing the state of matter as it has to work against the force of attraction of molecules.
Question 4. Suggest a method to liquefy atmospheric gases.
Answer: The gases can be converted into liquids by bringing its particles closer so atmospheric gases can be liquefied either by decreasing temperature or by increasing pressure.
In text questions page number 10
Question 1. Why does a desert cooler cool better on a hot dry day?
Answer: A desert cooler cools better on a hot dry day because on a hot dry day temperature is high and humidity is less which helps in better evaporation. Due to the higher rate of evaporation it gives better cooling effect.
Question 2. How does the water kept in an earthen pot (matka) become cool during summer?
Answer: The water kept in an earthen pot (matka) becomes cool during summer because of the evaporation of water through the fine holes in the earthen pot which makes it cool.
Question 3. Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Answer: When we put some acetone, petrol or perfume it starts evaporating by using the energy from palm and leaving it cool.
Question 4. Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Answer: We are able to sip tea, milk faster from a saucer because it has larger surface area than the cup, In larger surface area rate of evaporation is faster due to which tea or milk cools rapidly.
Question 5. What type of clothes should we wear in summer?
Answer: During summer we should wear cotton cloths because they are good absorber of water. As Cotton cloths absorbs sweat rapidly resulting in higher rate of evaporation Due to which our body feels cool.
Question 1. Convert the following temperatures to the Celsius scale.
(a) 293 K (b) 470 K.
Answer: To concert Kelvin scale into Celsius scale we have to subtract 273 so (a) 293K=293-273=20 ºC, (b) 470K=470-273=197 ºC
Question 2. Convert the following temperatures to the Kelvin scale.
(a) 25°C (b) 373°C.
Answer: To concert Celsius scale into Kelvin scale we have to add 273 so (a) 25 ºC=25+273=298K, (b) 373 ºC=373+273=646K
Question 3. Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
Answer: (a) Naphthalene balls undergo sublimation due to which it converts directly into vapours and disappear into air without leaving any solid.
(b) The particles of perfume diffuse rapidly into the air and its smell can be felt while sitting several metres away.
Question 4. Arrange the following substances in increasing order of forces of attraction between the particles— water, sugar, oxygen.
Answer: The force of attraction between the particles increases as we go from liquid to gas so the required order is: Oxygen<water<sugar.
Question 5. What is the physical state of water at (a) 25°C (b) 0°C (c) 100°C?
Answer: (a) Liquid, (b) Solid and (c) gas.
Question 6. Give two reasons to justify
(a) Water at room temperature is a liquid.
(b) an iron almirah is a solid at room temperature.
Answer: (a) water at room temperature is liquid because at this temperature (i)it has fixed volume and (ii)it can flow.
(B) An iron almirah is solid at room temperature because at this temperature (i) it has definite shape along with fixed volume and (ii) It can not flow like water and hence does not possess fluidity.
Question 7. Why is ice at 273 K more effective in cooling than water at the same temperature?
Answer: At 273K or 0 ºC the ice will give more cooling than water because it can absorb more heat than water due to its latent heat of fusion.
Question 8. What produces more severe burns, boiling water or steam?
Answer: Steam will produce more severe burns than boiling water because steam has more heat energy than water due to its latent heat of vaporisation.
Question 9. Name A, B, C, D, E and F in the following diagram showing change in its state
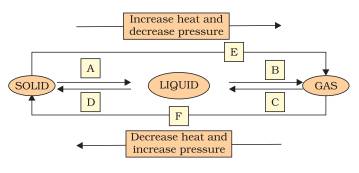
(i) A->Fusion
(ii) B->Vaporisation
(iii) C->Condensation
(iv) D->Solidification
(v) E->Sublimation
(vi) F->Sublimation
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.