
Ncert Solutions for class 10 subject Science Chapter 1 Chemical Reactions And Equationsin pdf Best Free NCERT Solutions for class 1 to 12 in pdf NCERT Solutions, cbse board, Science, ncert Solutions for Class 10 Science, class 10 Science ncert solutions, Chemical Reactions And Equations, Class 10, ncert solutions chapter 1 Chemical Reactions And Equations, class 10 Science, class 10 Science ncert solutions, Science ncert solutions class 10, Ncert Solutions Class 10 Science Chapter 1 Chemical Reactions And Equations
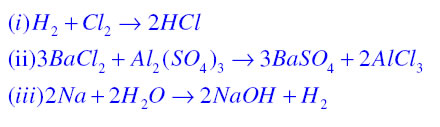
Answers:
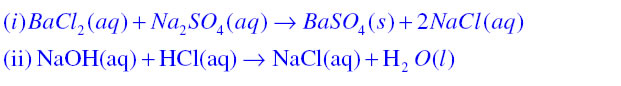 Answer:
Answer:
(i)The substance ‘X’ which is used in white washing is calcium oxide or quick lime and its formula is CaO.
(i)The reaction involved is
![]() Answer:
Answer:
The chemical reaction involved in the electrolysis of water is ![]()
From the above reaction it is clear that the amount of hydrogen gas and the oxygen gas formed are always in the ratio of 2:1. Therefore the volume of gas collected in the first test tube is double the volume of the gas collected in second test tube.Answer:
When an iron nail dipped in the copper sulphate solution than iron displaces copper from the copper sulphate because iron is more reactive than copper. Therefore the colour of the copper sulphate solution changes.
The reaction involved is
![]() Answer:
Answer:
![]() Answer:
Answer:
(i)The substance oxidised is sodium and the substance reduced is oxygen gas.
(ii) The substance oxidised is Hydrogen gas and the substance reduced is copper oxide.
Answer: (i)
Answer: (d)
Answer: (a)
Question 4. What is a balanced chemical equation? Why should chemical equations be balanced?
Answer:
If in a chemical equation the total number of atoms in the reactant side are equal to the total number of atoms in the product side then the chemical reaction is said to be balanced. For example
![]()
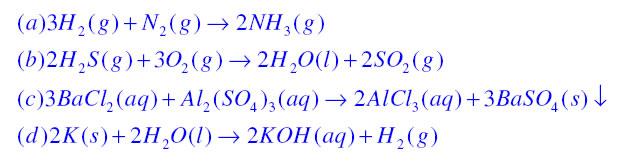 < align="justify" style="color:#0033FF; line-height:30px; font-size:18px;">< style="text-align:justify; font-size:16px; color:#0033FF; line-height:30px;">
< align="justify" style="color:#0033FF; line-height:30px; font-size:18px;">< style="text-align:justify; font-size:16px; color:#0033FF; line-height:30px;">
Answer:
Balanced chemical equations are
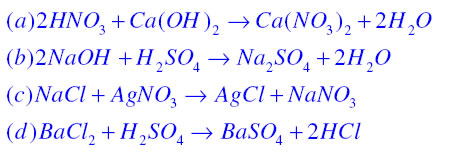
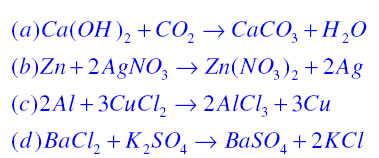
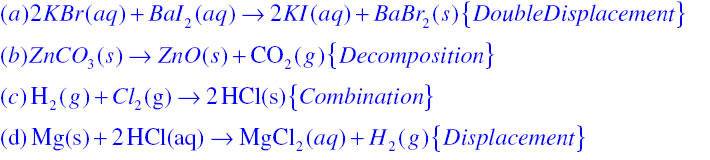 < align="justify" style="color:#0033FF; line-height:30px; font-size:18px;">< style="text-align:justify; font-size:16px; color:#0033FF; line-height:30px;">
< align="justify" style="color:#0033FF; line-height:30px; font-size:18px;">< style="text-align:justify; font-size:16px; color:#0033FF; line-height:30px;">
Answer:
Exothermic Reaction: A chemical reaction in which heat is given out is known as exothermic reaction. For example
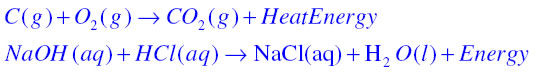
Answer:
Respiration is considered as an exothermic reaction because in respiration oxidation of glucose takes place which produces heat energy. This is shown in following chemical equation
![]()
Answer:
In Decomposition reaction a single substance decomposes to form two or more substances which is exact opposite of Combination reaction in which two or more reactants combine to form a single product that is why decomposition reactions called the opposite of combination reactions. For examples
Decomposition reaction:
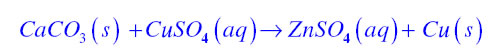
![]()
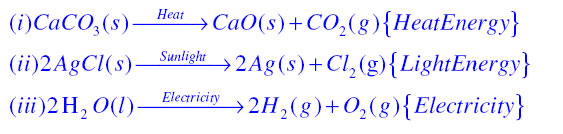
Answer:
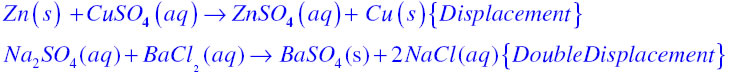
In displacement reaction a more reactive element replaces a less reactive element from its compound while in double displacement reaction exchange of ions takes place for example
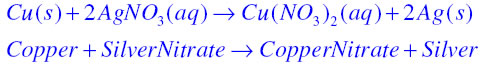
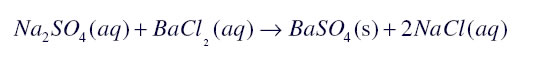
Answer:
Oxidation Reaction: It is a chemical reaction in which gain of oxygen or loss of hydrogen takes place. For example in the first reaction copper is oxidised to become copper oxide and in second Magnesium is oxidised to become Magnesium Oxide.

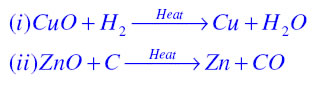
Answer: The shiny brown coloured element is copper and the black coloured compound formed is Copper oxide
![]()
Answer: Oil and fat containing food items flushed with nitrogen because nitrogen acts as an antioxidant and it prevent them from being oxidised.
< align="justify" style="color:#0033FF; line-height:30px; font-size:18px;">< style="text-align:justify; font-size:16px; color:#0033FF; line-height:30px;"> Answer:
Corrosion: It is the process in which metals are slowly eaten up by the action of air moisture or chemicals. For example rusting is a form of corrosion in which iron is eaten up by the action of air and moisture and a reddish brown coating of iron oxide is formed as shown in the following chemical reaction.
![]()
Concepts: Chemical Reactions and equations
< style="color:#002060">Chemical Equations: It is the symbolic representation of a chemical reaction where the reactant entities are given on the left hand side and the product entities on the right hand side of the equation. In complete chemical equation all reactants and products are written along with their physical state. A chemical equation must be balanced so that it can follow the law of conservation of mass. For example
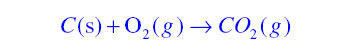
Balanced Chemical equation:If in a chemical equation the total number of atoms in the reactant side are equal to the total number of atoms in the product side then the chemical reaction is said to be balanced.
![]()
Combination Reaction:If in a Chemical equation, two or more reactants combine to form a single product then it is known as combination reaction.
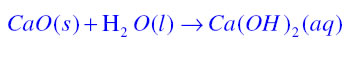
Decomposition reaction:If in a chemical reaction a single substance decomposes to form two or more substances then it is known as decomposition reaction.
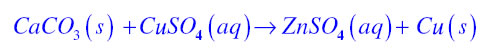
Displacement Reaction:If in a chemical equation, more reactive elements displaces a less reactive element from its compound then it is known as displacement reaction.
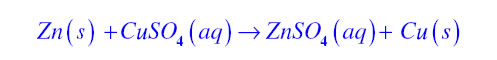
Double displacement reaction:If in a chemical reaction exchange of ions takes place then it is known as double displacement reaction
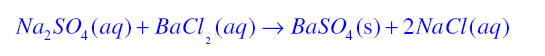
Precipitation Reaction:A white coloured substance which is insoluble in water also known as precipitate is formed during a chemical reaction then the reaction is known as precipitation reaction
![]()
In this reaction Barium sulphate is a white colour precipitate.
Oxidation Reaction:It is a chemical reaction in which gain of oxygen or loss of hydrogen takes place. For example in the following reaction copper is oxidised to become copper oxide.
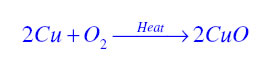
Reduction Reaction:It is a chemical reaction in which loss of oxygen or gain of hydrogen takes place. For example in the following reaction copper oxide is reduced to become copper.
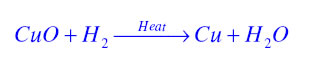
Redox Reaction or Oxidation-Reduction reaction:The chemical reaction in which both oxidation and reduction takes place is known as Redox reaction. For example in the following reaction Hydrogen is oxidised to become water and copper oxide is reduced to become copper.

Corrosion:It is the process in which metals are slowly eaten up by the action of air moisture or chemicals. For example rusting is a form of corrosion in which iron is eaten up by the action of air and moisture and a reddish brown coating of iron oxide is formed as shown in the following chemical reaction.
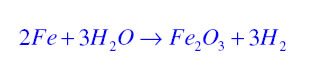
Rancidity:When the substance containing oils and fats are exposed to air they get oxidised and become rancid due to which their smell, taste and colour change. This process is known as rancidity. For example when a when butter is kept open for a long time then its smell and taste gets changed.
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.