
Here we are providing CBSE Previous Year Question Papers Class 6 to 12 solved with soutions Science Question Paper For Class 10 2018 2019 2020 CBSE Board NCERT Books class 10 Science sample paper 2020 solved, sample paper class 10 Science, cbse class 10 Science question paper 2018, sample paper class 10 Science 2019, Science question paper for class 10, cbse previous year question papers class 10 Science, cbse sa Practice of previous year question papers and sample papers protects each and every student to score bad marks in exams.If any student of CBSE Board continuously practices last year question paper student will easily score high marks in tests. Fortunately earlier year question papers can assist the understudies with scoring great in the tests. Unraveling previous year question paper class 10 Science is significant for understudies who will show up for Class 10 Board tests.
Question 1: (Marks 1)
Covalent compounds have low melting and boiling point. Why ?
Answer :
Due to weak intermolecular forces.
Question 2: (Marks 1)
How many metals are present in second period of periodic table ?
Answer :
Two / Lithium and Beryllium
Question 3: (Marks 4)
Geothermal energy is the energy produced by the heat of molten rocks formed in the deeper hot regions of the earth’s crust. This energy is harnessed to generate electricity. When water is made to flow deep underground in the rocks it returns as steam (or hot water, which is later converted to steam) to drive a turbine on an electric power generator.
In India, exploration and study of geothermal fields started in 1970. The Geological Survey in India has identified 350 geothermal energy locations in the country. The most promising of these is in Puga valley of Ladakh. The estimated potential for geothermal energy in India is about 10000 MW. There are seven geothermal provinces in India namely the Himalayas, Sohna, West coast, Cambay, Son-Narmada-Tapi; Godavari and Mahanadi. Most power stations in India produce Alternating Current (A.C).
(a) What are geothermal energy hot-spots ? (Marks 1)
(b) Name two countries, other than India, where power plants based on geothermal energy are operational. (Marsk 1)
(c) Name the phenomenon that explains the working of an electric generator. (Marks 1)
(d) State an important advantage of using AC over DC. (Marks 1)
Answer :
(a) Are deeper hot regions of earth’s crust where molten rocks are formed.
(b) New Zealand / United States of America / China/Indonesia, Philippines / Turkey/ New Mexico.
(Any two)
(c) Electromagnetic Induction.
(d) In case of A.C. transmission of power/electricity takes place without much loss of energy.
Question 4: (Marks 4)
Thyroid gland is a bilobed structure situated in our neck region. It secretes a hormone called thyroxine. Iodine is necessary for the thyroid gland to make thyroxine. Thyroxine regulates carbohydrate, protein and fat metabolism in the body. It promotes growth of body tissues also. When there is an excess of thyroxine in the body, a person suffers from hyperthyroidism and if this gland is underactive it results in hypothyroidism. Hyperthyroidism is diagnosed by blood tests that measure the levels of thyroxine and Thyroid Stimulating Hormone (TSH). Hypothyroidism is caused due to the deficiency of iodine in our diet resulting in a disease called goitre. Iodised salt can be included in our diet to control it.
(a) Where is thyroid gland situated in our body ? (Marks 1)
(b) State the function of thyroxine in human body. (Marks 1)
(c) What is hyperthyroidism ? (Marks 1) (Marks 1)
(d) How can we control hypothyroidism ? (Marks 1)
Answer :
(a) In the neck region
(b) Thyroxine regulates carbohydrate, proteins and fat metabolism in the body./ It promotes growth of body tissue.
(c) Excess of secretion of throxine in the body /overactivity of the thyroid gland
(d) Can be controlled by including iodised salt in our diet.
(or any other relevant answer)
Question 5: (Marks 1)
Consider the following reasons for the reddish appearance of the sun at the sunrise or the sunset :
A. Light from the sun near the horizon passes through thinner layers of air.
B. Light from the sun covers larger distance of the earth’s atmosphere before reaching our eyes.
C. Near the horizon, most of the blue light and shorter wavelengths are scattered away by the particles.
D. Light from the sun near the horizon passes through thicker layers of air.
The correct reasons are
| (a) A and C only | (b) B, C and D |
| (c) A and B only | (d) C and D only |
OR
Person suffering from cataract has
(a) elongated eyeball
(b) excessive curvature of eye lens
(c) weakened ciliary muscles
(d) opaque eye lens (Marks 1)
Answer :
(b) / B,C and D
OR
(d) /Opaque eye lens
Question 6: (Marks 1)
The maximum resistance which can be made using four resistors each of 2 Ω is
(a) 2 Ω
(b) 4 Ω
(c) 8 Ω
(d) 16 Ω
Answer :
(c) / 8 Ω
Question 7: (Marks 1)
A student plots V-I graphs for three samples of nichrome wire with resistances R1, R2 and R3. Choose from the following the statement that holds true for this graph.
(a) R1 = R2 = R3
(b) R1 > R2 > R3
(c) R3 > R2 > R1
(d) R2 > R1 > R3
Answer :
(d) / R2 > R1 > R3
Question 8: (Marks 1)
Which of the following are water intensive crops ?
(a) Wheat and rice
(b) Wheat and sugarcane
(c) Sugarcane and rice
(d) Wheat and gram
OR
The most poisonous product formed by incomplete combustion of fossil fuels is
(a) Carbon dioxide
(b) Nitrogen dioxide
(c) Carbon monoxide
(d) Sulphur dioxide
Answer :
(c)/ Sugarcane and rice
OR
(c) / Carbon monoxide
Question 9: (Marks 1)
Bandharas and Tals are age old water harvesting concepts / structures found in
(a) Bihar
(b) Maharashtra
(c) Tamil Nadu
(d) Rajasthan
Answer :
(b) / Maharashtra
Question 10: (Marks 1)
Answer :
(d) / x= Physical state of KClO3 and KCl
y = Reaction condition
z= Physical state of O2
Question 11: (Marks 1)
A visually challenged student, has to perform a lab test to detect the presence of acid in a given solution. The acid-base indicator preferred by him will be :
| (a) Blue litmus | (b) Clove oil |
| (c) Red cabbage extract | (d) Hibiscus extract |
Answer :
(b) / Clove oil
Question 12: (Marks 1)
On the basis of electronic configuration of 59X, the group number and period of the element ‘X’ is :
| (a) Group 15 period 2 | (b) Group 13 period 2 |
| (c) Group 9 period 5 | (d) Group 13 period 5 |
OR
An element ‘X’ with atomic number 11 forms a compound with element ‘Y’ with atomic number 8. The formula of the compound formed is
| (a) XY | (b) X2Y |
| (c) XY2 | (d) X2Y3 |
Answer :
(b) / Group 13 period 2
OR
(b) / X2Y
(a) Both (A) and (R) are true and (R) is correct explanation of the assertion.
(b) Both (A) and (R) are true but (R) is not the correct explanation of the assertion.
(c) (A) is true but (R) is false.
(d) (A) is false but (R) is true.
Question 13: (Marks 1)
Assertion (A) : Ethanoic acid is also known as glacial acetic acid.
Reason (R) : The melting point of pure ethanoic acid is 290 K and hence it often freezes during winters in cold climates.
Answer :
(a) / Both (A) and (R) are true and (R) is the correct explanation of the assertion.
Question 14: (Marks 1)
Assertion (A) : The metals and alloys are good conductors of electricity.
Reason (R) : Bronze is an alloy of copper and tin and it is not a good conductor of electricity.
Answer :
(d) / (A) is false, but (R) is true.
Question 15: (Marks 3)
A compound ‘A’ is used in the manufacture of cement. When dissolved in water, it evolves a large amount of heat and forms compound ‘B’.
(i) Identify A and B.
(ii) Write chemical equation for the reaction of A with water.
(iii) List two types of reaction in which this reaction may be classified.
Answer :
(i) A = CaO / Quick lime/ Calcium oxide
B = Ca(OH)2 / Slaked lime / Calcium hydroxide
(ii) CaO + H2O → Ca(OH)2 + heat or energy
(iii) Combination reaction
Exothermic reaction
Question 16: (Marks 3)
Give reasons for the following :
(i) Only one half of water molecule is shown in the formula of Plaster of Paris.
(ii) Sodium hydrogen carbonate is used as an antacid.
(iii) On strong heating, blue coloured copper sulphate crystals turn white. (Marks 3)
OR
(i) Draw a labelled diagram to show the preparation of hydrogen chloride gas in laboratory.
(ii) Test the gas evolved first with dry and then with wet litmus paper. In which of the two cases, does the litmus paper show change in colour ?
(iii) State the reason of exhibiting acidic character by dry HCl gas / HCl solution. (Marks 3)
Answer :
(i) 2 formula units of CaSO4 /Calcium sulphate share 1 molecule of water of crystallization.
(ii) due to its alkaline nature .
(iii) CuSO4 .5H2O CuSO4 + 5H2O (Blue) (white) / Due to loss of water of crystallization.
OR
(ii) Wet litmus paper
(iii) HCl solution , it is due to the formation of H+ ion on in the water / H3O+ (Hydronium ions)
Question 17: (Marks 3)
From the elements 1919A, 2814B, 168C and 4018D identify :
(a) the most electro positive element.
(b) a noble gas.
(c) a metalloid.
(d) an element which will gain 2 electrons to attain nearest noble gas configuration.
(e) formula of compound formed between A and C.
(f) elements belonging to same period. (Marks 3)
Answer :
(a) A
(b) D
(c) B
(d) C
(e) A2C
(f) B & D
Question 18: (Marks 3)
(a) Construct a terrestrial food chain comprising four trophic levels.
(b) What will happen if we kill all the organisms in one trophic level ?
(c) Calculate the amount of energy available to the organisms at the fourth trophic level if the energy available to the organisms at the second trophic level is 2000 J. (Marks 3)
OR
(a) Complete the following table :
| Oxygen | Ozone | |
| Formula | (i) ____________ | (ii) ____________ |
| Benefits to biotic component | (iii) ____________ | (iv) ____________ ____________ ____________ |
(b) How is ozone formed at the higher levels of atmosphere ? (Marks 3)
Answer :
(a) Grass → Grass hopper → Frog → Snake
(Or any other relevant example)
(b) Transfer of food energy to the next higher level will not take place , then the organisms of the upper trophic levels will be affected , increase in the population of the organisms belonging to the previous trophic level / imbalance in the food chain.
[If calculation of the amount of energy is not shown , deduct ½ mark .]
OR
(a) (i) O2
(ii) O3
(iii) Breathing /Respiration
(iv) Absorbs harmful ultra violet (UV) radiations.
Question 19: (Marks 3)
Answer :
Secretions | Functions |
| (a) mucus | (d)Protects the inner lining of stomach from the acid / softening of food |
| (b) HCl(Hydrochloric acid) | (e)Provides the acidic medium for action of enzyme / Kill the germs. |
| (c) Pepsin | (f) Digest proteins |
(Note : a,b and c may in any order but there function must match / be given along with the secretion.
Question 20: (Marks 3)
Explain giving an example how the following provide evidences in favour of evolution in organisms.
| (i) Homologous organs | (ii) Fossils |
Answer :
(i) Homologous organs: Mammals have forelimbs as do birds, reptiles and amphibians .The basic structure of the limbs is similar though it has been modified to perform different functions in various vertebrates. Therefore these are homologus organs.
(ii) Fossils: Study of fossils of Archeopteryx / Dinosaurs show the presence of feathers used for insulation in cold weather but later became useful for flight. So birds have evolved from reptiles.
Question : 21 (Marks 3)
What are chromosomes ? Explain how in sexually reproducing organisms the number of chromosomes in the progeny is maintained.
Answer :
► Chromosomes are thread like structures present in nucleus containing genetic material / DNA
► Number of chromosomes are reduced to half during gametes / germ cell formation . After fertilization of germ cells the number of chromosomes is maintained in progeny.
Question 22: (Marks 3)
What happens after refraction, when :
(i) a ray of light parallel to the principal axis passes through a concave lens ?
(ii) a ray of light falls on a convex lens while passing through its principal focus ?
(iii) a ray of light passes through the optical centre of a convex lens ?
Answer :
Question 23: (Marks 3)
Two coils of insulated copper wire are wound over a non-conducting cylinder as shown. Coil 1 has comparative large number of turns. State your observations, when
(i) Key K is closed.
(ii) Key K is opened.
Give reason for each of your observations.
Answer :
(i) Galvanometer(G) shows deflection (for very short time)
(ii) Galvanometer (G) shows deflection for a very short time in opposite direction to the previous observation.
Common Reason: Due to variation in current flowing through coil 1, magnetic field associated with coil 2 changes. Due to which an induced current will generate consequently galvanometer shows momentry defelection.
Question 24: (Marks 3)
(a) List two causes of hypermetropia.
(b) Draw ray diagrams showing (i) a hypermetropic eye and (ii) its correction using suitable optical device.
OR
(a) State the relation between colour of scattered light and size of the scattering particle.
(b) The apparent position of an object, when seen through the hot air, fluctuates or wavers. State the basic cause of this observation.
(c) Complete the path of white light when it passes through two identical prisms placed as shown :
Answer :
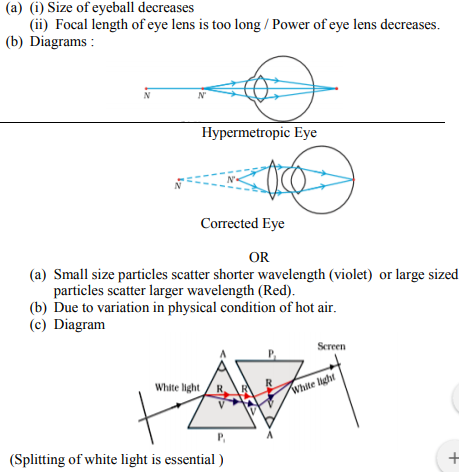
Question 25: (Marks 5)
(a) How is the method of extraction of metals high up in the reactivity series different from that for metals in the middle ? Why cannot the same process be applied for them ? Name and explain the process of extraction of sodium.
(b) Draw a labelled diagram of electrolytic refining of copper.
OR
What happens when (Write the balanced equation involved) –
(i) Copper is heated in air ?
(ii) Aluminium oxide is reacted with hydrochloric acid ?
(iii) Potassium reacts with water ?
(iv) Cinnabar is heated in air ?
(v) Aluminium oxide reacts with sodium hydroxide ?
Answer :
(a)
► Metals high up in reactivity series cannot be obtained from their compounds by heating with carbon as carbon can not reduce the oxides of these elements while those in the middle of the reactivity series are extracted first by converting their sulphides or carbonates into oxides and then reducing by Carbon .
► It is because these metals have high affinity for oxygen than Carbon .
► Electrolytic reduction
► Sodium is obtained from its molten chloride by passing electricity.
► at Cathode : Na+ + e- Na
at Anode : 2Cl Cl2+ 2e-
Question 26: (Marks 5)
(a) What is a homologous series ? Explain with an example.
(b) Define the following terms giving one example of each.
(i) Esterification
(ii) Addition reaction
Answer :
Question 27: (Marks 5)
(a) Describe the structure and function of the basic filtering unit of kidney.
(b) List two factors on which reabsorption of water from urine depends ?
Answer :
(a) ► Nephron
► Structure : Cluster of blood capillaries / glomerulus is associated with cup shaped structure called Bowman’s capsule, which leads to coiled tubular part of Nephron.
Function : Collects the filterate and reabsorbs useful substances like glucose,amino acids, salts and water from filterate and forms urine.
(b) Amount of excess water in the body
Amount of wastes dissolved
Question 28: (Marks 5)
(a) List three different categories of contraception methods.
(b) Why has Government of India prohibited prenatal sex determination by law ? State its benefits in the long run.
(c) Unsafe sexual act can lead to various infections. Name two bacterial and two viral infections caused due to unsafe sex.
OR
(a) In the female reproductive system of human beings, state the funtions of
(i) ovary (ii) oviduct
(b) Mention the changes which the uterus undergoes, when
(i) it has to receive a zygote
(ii) no fertilization takes place.
(c) State the function of placenta
Answer :
(a)
► Chemical Method
► Barrier Method
► Surgical Method
(b) Increase in female foeticide / Declining child sex ratio
(Any One)
Benefit : Maintaining male-female sex ratio for a healthy society
(c) Bacterial → Gonorrhoea Syphilis
Viral → Warts
AIDS
OR
(a) (i) Ovary→ Production of female germ cell/egg
Production of hormone – estrogen
(Any one)
(i) Oviduct→ Site of fertilization
(b) (i) Thickening of the uterus lining
(ii) Wall of uterus breaks/Menstruation occurs.
(c) Providing the nutrition / O2/to the developing embryo /foetus or removal of waste from the fetus.
Question 29: (Marks 5)
(a) Find the ratio of resistances of two copper rods X and Y of lengths 30 cm and 10 cm respectively and having radii 2 cm and 1 cm respectively.
(b) A current of 500 mA flows in a series circuit containing an electric lamp and a conductor of 10 Ω when connected to 6 V battery. Find the resistance of the electric lamp.
Answer :
Question 30: (Marks 5)
(a) A concave mirror of focal length 10 cm can produce a magnified real as well as virtual image of an object placed in front of it. Draw ray diagrams to justify this statement.
(b) An object is placed perpendicular to the principal axis of a convex mirror of focal length 10 cm. The distance of the object from the pole of the mirror is 10 cm. Find the position of the image formed.
OR
(a) Define the following terms :
(i) Power of a lens
(ii) Principal focus of a concave mirror
(b) Write the relationship among the object distance (u), image distance (v) and the focal length (f) of a
(i) Spherical lens
(ii) Spherical mirror
(c) An object is placed at a distance of 10 cm from optical centre of a convex lens of focal length 15 cm. Draw a labelled ray diagram to show the formation of image in this case.
Answer :
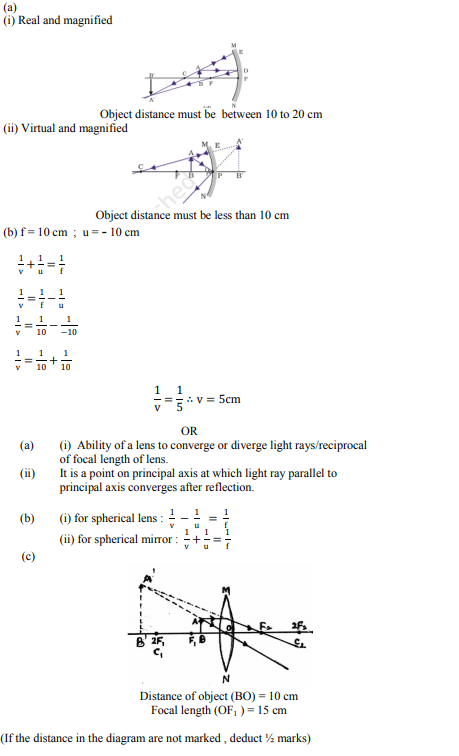
class 10 Science sample paper 2020 solved, sample paper class 10 Science, cbse class 10 Science question paper 2018, sample paper class 10 Science 2019, Science question paper for class 10, cbse previous year question papers class 10 Science, cbse sample paper for class 10 Science, cbse class 10 Science question paper 2019, class 10 Science sample paper 2019 solved, cbse class 10th Science question paper, 10th Science question paper, sample paper class 10 Science 2020, Science sample paper class 12, cbse question paper for class 9 Science, cbse Science sample paper, cbse 10th class Science
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.