
Here we are providing CBSE Previous Year Question Papers Class 6 to 12 solved with soutions CBSE Previous Year Question Papers Class 10 Science Chapter wise 2018 19 20 class 10 Science sample paper 2020 solved, sample paper class 10 Science, cbse class 10 Science question paper 2018, sample paper class 10 Science 2019, Science question paper for class 10, cbse previous year question papers class 10 Science, cbse sa Practice of previous year question papers and sample papers protects each and every student to score bad marks in exams.If any student of CBSE Board continuously practices last year question paper student will easily score high marks in tests. Fortunately earlier year question papers can assist the understudies with scoring great in the tests. Unraveling previous year question paper class 10 Science is significant for understudies who will show up for Class 10 Board tests.
Question 1: (Marks 1)
How are covalent bonds formed ?
Answer :
Covalent bonds are formed by sharing of electron pair /pairs between two atoms.
Question 2: (Marks 1)
Define electropositivity.
OR
The atomic radii of first group elements are given below :
Group-I element | Atomic Radii (pm) |
Na | 86 |
K | 231 |
Rb | 244 |
Ca | 282 |
State the reason behind the observed trend in the above elements.
Answer :
Tendency of an element to lose electrons.
OR
Atomic radii increases from Na to Cs due to addition of new shells.
Question 3: (Marks 4)
The Tehri dam is the highest dam in India and one of the highest in the World. The Tehri dam withholds a reservoir of capacity 4.0 km3 and surface area 52 km2 . It is used for irrigation, municipal water supply and the generation of 1000 MW of hydro electricity.
The Tehri Dam has been the object of protests. Environment activist Shri Sunder Lal Bahuguna led the “Anti Tehri Dam Movement” from 1980s to 2014. The protest was against the displacement of town inhabitants and environmental consequences of the weak ecosystem. The relocation of more than 1,00,000 people from the area has led to protracted legal battles over resettlement rights and ultimately resulted in the delayed completion of the project.
(a) How is hydropower harnessed ? (Marks 1)
(b) Define 1 MW. (Marks 1)
(c) Mention two disadvantages of constructing Tehri Dam. (Marks 1)
(d) What happens when water from great heights is made to fall on blades of turbine ? (Marks 1)
Answer :
(a) Hydropower is harnessed by converting the potential energy of falling water from a height into electricity.
(b) It is the power developed when 106 J of work is done per second. / 1MW = 106 watts.
(c) Loss of agricultural land / displacement of a large number of peasants and tribals/ destruction of ecosystem. (any two)
(d) The blades of turbine move the armature of a generator with high speed to generate electricity.
Question 4: (Marks 4)
Questions numbers 4(a) to 4(d) are based on table given below. Study the table in which the levels of Thyroid Stimulating Hormone (TSH) in women are given and answer the questions that follow on the basis of understanding of the following paragraph and the related studied concepts.
Age Range | Normal (mU/L) | Low (mU/L) |
18 – 29 years | 0.4 – 2.34 mU/L | < 0.4 mU/L |
30 – 49 years | 0.4 – 4.0 mU/L | < 0.4 mU/L |
50 – 79 years | 0.46 – 4.68 mU/L | < 0.46 mU/L |
Women are at greater risk for developing abnormal TSH levels during menstruation, while giving birth and after going through menopause. Around 5% of women in the United States have some kind of thyroid problem compared to 3% of men. Despite claims that high TSH increases your risk for heart disease, a 2013 study found no link between high TSH and heart diseases. But a 2017 study showed that older women are especially at risk for developing thyroid cancer if they have high TSH levels along with thyroid nodules.
(a) A 35 year old woman has TSH level 6.03 mU/L. What change should she bring in her diet to control this level ? (Marks 1)
(b) When do women face a greater risk of abnormal TSH level ? (Marks 1)
(c) State the consequence of low TSH level. (Marks 1)
(d) Name the mineral that is responsible for synthesis of hormone secreted by thyroid gland. (Marks 1)
Answer :
(a) She should monitor iodine intake in her diet.
(b) During menstruation / during pregnancy and after going through menopause.
(any two)
(c) Low TSH level leads to swelling of neck region / disease called goiter.
(d) Iodine
Question 5: (Marks 1)
The sky appears dark to passengers flying at very high altitudes mainly because :
(a) Scattering of light is not enough at such heights.
(b) There is no atmosphere at great heights.
(c) The size of molecules is smaller than the wavelength of visible light.
(d) The light gets scattered towards the earth.
Answer :
(a) / Scattering of light is not enough at such heights
Question 6: (Marks 1)
Answer :
(c) / 2 A
Question 7: (Marks 1)
Answer :
(a) / 2 Ω
Question 8: (Marks 1)
A diagram of traditional water harvesting system is given below :
The statement which defines the system and its parts is
(a) This is an ideal setting of the Khadin system and A = Catchment area; B = Saline area & C = Shallow dugwell
(b) This is an ideal setting of the Shallow dugwell system and A = Catchment area; B = Saline area and C = Khadin
(c) This is an ideal setting of Catchement area and A = Khadin, B = Saline area and C = Shallow dugwell
(d) This is showing Saline area and A = Catchment area; B = Khadin and C = Shallow dugwell
OR
The major ill effect of mono culture practice in forests is on the
(a) biodiversity which faces large destruction
(b) local people whose basic needs can no longer be met from such forests
(c) industries
(d) forest department
Answer :
(a) /This is an ideal setting of the Khadin system and A= catchment area; B= Saline area ; C=Shallow dugwell.
OR
(a) / biodiversity which faces large destruction.
Question 9: (Marks 1)
Several factories were pouring their wastes in rivers A and B. Water samples were collected from these two rivers. It was observed that sample collected from river A was acidic while that of river B was basic. The factories located near A and B are
(a) Soaps and detergents factories near A and alcohol distillery near B.
(b) Soaps and detergents factories near B and alcohol distillery near A.
(c) Lead storage battery manufacturing factories near A and soaps and detergents factories near B.
(d) Lead storage battery manufacturing factories near B and soaps and detergents factories near A.
Answer :
(c) / Lead storage battery manufacturing factories near A and soaps and detergents factories near B.
Question 10: (Marks 1)
In which of the following, the identity of initial substance remains unchanged ?
(a) Curdling of milk
(b) Formation of crystals by process of crystallisation
(c) Fermentation of grapes
(d) Digestion of food
Answer :
(b) / Formation of crystals by process of crystallisation.
Question 11: (Marks 1)
An aqueous solution ‘A’ turns phenolphthalein solution pink. On addition of an aqueous solution ‘B’ to ‘A’, the pink colour disappears. The following statement is true for solution ‘A’ and ‘B’.
(a) A is strongly basic and B is a weak base
(b) A is strongly acidic and B is a weak acid.
(c) A has pH greater than 7 and B has pH less than 7.
(d) A has pH less than 7 and B has pH greater than 7.
Answer :
(c) / A has pH greater than 7 and B has pH less than 7.
Question 12: (Marks 1)
An element ‘X’ is forming an acidic oxide. Its position in modern periodic table will be
(a) Group 1 and Period 3
(b) Group 2 and Period 3
(c) Group 13 and Period 3
(d) Group 16 and Period 3
OR
Consider the following statements about an element ‘X’ with number of protons 13.
(A) It forms amphoteric oxide
(B) Its valency is three
(C) The formula of its chloride is XCl3
The correct statements(s) is/are
(a) only (A)
(b) only (B)
(c) (A) and (C)
(d) (A), (B) and (C)
Answer :
(c) / A has pH greater than 7 and B has pH less than 7. 1 1 12. (d) / Group 16 and Period 3
OR
(d) / (A), (B) & (C)
(a) Both (A) and (R) are true and (R) is correct explanation of the assertion.
(b) Both (A) and (R) are true but (R) is not the correct explanation of the assertion.
(c) (A) is true but (R) is false.
(d) (A) is false but (R) is true.
Question 13: (Marks 1)
Assertion (A) : Following are the members of a homologous series : CH3OH, CH3CH2OH, CH3CH2CH2OH
Reason (R) : A series of compounds with same functional group but differing by – CH2 – unit is called a homologous series.
Answer :
(a) / Both (A) and (R) are true and (R) is the correct explanation of the assertion.
Question 14:
Assertion (A) : Alloys are commonly used in electrical heating devices like electric iron and heater.
Reason (R) : Resistivity of an alloy is generally higher than that of its constituent metals but the alloys have low melting points then their constituent metals.
Answer :
(c) / A is true but R is false.
Question 15:
Mention with reason the colour changes observed when :
(i) silver chloride is exposed to sunlight.
(ii) copper powder is strongly heated in the presence of oxygen.
(iii) a piece of zinc is dropped in copper sulphate solution.
Answer :
(i) White to grey
Reason : Silver chloride decomposes to produce silver and chlorine.
(ii) Brown to black
Reason : Copper oxide is produced on heating.
(iii) Blue to colourless
Reason : Zinc Sulphate is formed.
Question 16:
Complete and balance the following chemical equations :
(i) NaOH(aq) + Zn(s) →
(ii) CaCO3(s) + H2O(l) + CO2(g) →
(iii) HCl(aq) + H2O(l) →
OR
During electrolysis of brine, a gas ‘G’ is liberated at anode. When this gas ‘G’ is passed through slaked lime, a compound ‘C’ is formed, which is used for disinfecting drinking water.
(i) Write formula of ‘G’ and ‘C’.
(ii) State the chemical equation involved.
(iii) What is common name of compound ‘C’ ? Give its chemical name.
Answer :
(i) 2NaOH(aq) + Zn(s) → Na2(Zn)O2(aq) + H2(g)
(ii) CaCO3(s)+H2O(l) + CO2(g) → Ca (HC03)2(aq)
(iii) HCl(aq) + H2O(l) → H3O(aq)+ + Cl(aq) −
Note : Deduct half marks if equations are not balanced.
OR
(i)G = Cl2
C = CaOCl2
(ii) Ca(OH)2 + Cl2 → CaOCl2 + H2O
(iii) Common name – Bleaching Powder
Chemical name – Calcium Oxychloride
Note : Give full credit for writing common name only
Question 17:
Answer :
(i) Category A / Li, Na, K
(ii) Because the physical as well as chemical properties of elements of category A,B and C are different.
(iii) No
Reason : Because Newlands’ law of octaves was applicable only upto calcium
Question 18:
(a) From the following group of organisms create a food chain which is the most advantageous for Human beings in terms of energy.
(b) State the possible disadvantage if the cereal plant is growing in soil rich in pesticides.
(c) Construct a food web using the organisms mentioned above.
OR
(a) Write two harmful effects of using plastic bags on the environment. Suggest alternatives to the usage of plastic bags.
(b) List any two practices that can be followed to dispose off the waste produced in our homes.
Answer :
(a) Cereal Plant → Human Beings.
(b) Pesticides being non-biodegradable accumulate progressively at each trophic level/ Leads to Biomagnification.
(a)
► Harmful effects of using plastic bags :
(i) They lead to land /water pollution when disposed improperly.
(ii) Burning of plastic would produce toxic gases/ air pollution.
(iii) Plastic bags can block the drainage system.
( or any other)
(any two)
► Alternatives to the usage of plastic bags:
(i) Use of cloth bags/ jute bags/ paper bags
(ii) Metal or glass containers.
(b)
(i) Segregation of biodegradable and non-biodegradable wastes for recycling / Segregation of dry and wet waste for recycling.
(ii) Reuse of already used items like glass bottles for storage. (iii) composting
(or any other)
(any two)
Question 19:
(a) State the role played by the following in the process of digestion.
(i) Enzyme trypsin
(ii) Enzyme lipase
(b) List two functions of finger like projections present in the small intestine.
Answer :
(a) (i) Enzyme trypsin : Helps in the digestion of proteins.
(ii) Enzyme lipase : Helps in the breaking down of emulsified fats.
(b) Two functions :
► Increase the surface area .
► Helps in absorption of digested food.
(Note : Full credit for the statement : Increase the surface area for the absorption of digested food).
Question 20:
(a) Classify the following as homologous or analogous pairs :
(i) Broccoli and Cabbage
(ii) Ginger and Raddish
(iii) Fore limbs of birds and lizard
(iv) Wings of a bat and Wings of a bird
(b) State the main feature that categorises a given pair of organs as homologous or analogous.
Answer :
(a) (i) Analogous
(ii) Analogous
(iii) Homologous
(iv) Analogous
(b) Homologous organs have similar origin and basic structure but perform different functions whereas Analogous organs have different basic structure but perform similar functions.
Question 21:
A green stemmed rose plant denoted by GG and a brown stemmed rose plant denoted by gg are allowed to undergo a cross with each other.
(a) List your observations regarding
(i) Colour of stem in their F1 progeny
(ii) Percentage of brown stemmed plants in F2 progeny if F1 plants are self pollinated.
(iii) Ratio of GG and Gg in the F2 progeny.
(b) Based on the findings of this cross, what conclusion can be drawn ?
Answer :
(a) (i) Green
(ii) 25 %
(iii) GG : Gg
1 : 2
(b) The traits which are expressed in F1 progeny are called dominant traits, whereas the traits which are unable to express themselves in F1 progeny but reappear in the F2 progeny are called recessive traits.
Question 22:
The diagram given below shows an object O and its image I.
Without actually drawing the ray diagram, state the following :
(i) Type of lens (Converging / Diverging)
(ii) Name two optical instruments where such an image is obtained.
(iii) List three characteristics of the image formed if this lens is replaced by a concave mirror of focal length ‘f’ and an object is placed at a distance ‘f/2’ in front of the mirror
Answer :
(i) Converging Lens
(ii) Magnifying Glass, Microscope
(iii) Three Characteristics of the image :
(a) Virtual (b) Erect (c) Magnified
Question 23:
Give reasons for the following :
(i) There is either a convergence or a divergence of magnetic field lines near the ends of a current carrying straight solenoid.
(ii) The current carrying solenoid when suspended freely rests along a particular direction.
(iii) The burnt out fuse should be replaced by another fuse of identical rating.
Answer :
(i) The strength of magnetic field is higher near the poles /ends of solenoid.
(ii) A current carrying solenoid behaves as a bar magnet.
(iii) If a fuse , with a defined rating , is replaced by one with a larger rating then the fuse wire will not burn even when a current greater than safe limit is flowing. As a result the electrical circuit / appliances will be damaged.
Question 24:
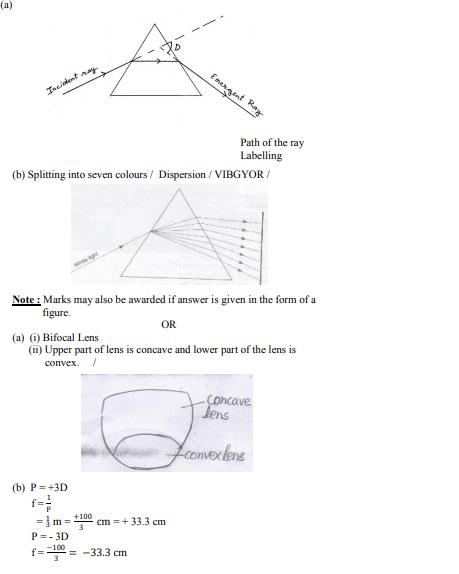
(a) With the help of labelled ray diagram show the path followed by a narrow beam of monochromatic light when it passes through a glass prism.
(b) What would happen if this beam is replaced by a narrow beam of white light ?
OR
(a) A person is suffering from both myopia and hypermetropia.
(i) What kind of lenses can correct this defect ?
(ii) How are these lenses prepared ?
(b) A person needs a lens of power + 3D for correcting his near vision and –3D for correcting his distant vision. Calculate the focal lengths of the lenses required to correct these defects.
Answer :
Question 25:
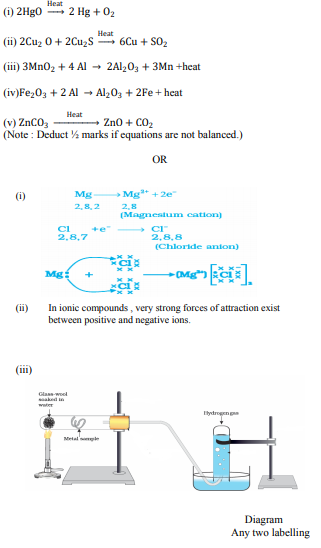
Write balanced chemical equations to explain what happens, when
(i) Mercuric oxide is heated.
(ii) Mixture of cuprous oxide and cuprous sulphide is heated.
(iii) Aluminium is reacted with manganese dioxide.
(iv) Ferric oxide is reduced with aluminium.
(v) Zinc carbonate undergoes calcination.
OR
(i) By the transfer of electrons, illustrate the formation of bond in magnesium chloride and identify the ions present in this compound.
(ii) Ionic compounds are solids. Give reasons.
(iii) With the help of a labelled diagram show the experimental set up of action of steam on a metal.
Answer :
Question 26:
(a) Compare soaps and detergents on the basis of their composition and cleansing action in hard water.
(b) What happens when ethanol is treated with sodium metal ? State the behaviour of ethanol in this reaction.
(c) Draw the structure of cyclohexane.
(d) Name the following compound.
Answer :
Question 27:
(a) Write the correct sequence of steps followed during journey of oxygen rich blood from lungs to various organs of human body.
(b) What happens when the system of blood vessels develop a leak ?
Answer :
bz
Question 28:
(a) Draw a diagram showing germination of pollen on stigma of a flower and mark on it the following organs/parts :
(i) Pollen Grain
(ii) Pollen tube
(iii) Stigma
(iv) Female germ cell
(b) State the significance of pollen tube.
(c) Name the parts of flower that develop after fertilization into
(i) Seed
(ii) Fruit
OR
(a) “Use of a condom is beneficial for both the sexes involved in a sexual act.” Justify this statement giving two reasons.
(b) How do oral contraceptive help in avoiding pregnancies ?
(c) What is sex selective abortion ? How does it affect a healthy society ?
(State any one consequence)
Answer :
(b) Pollen tube carries the male germ cell to reach the ovary and fuse with the female germ cell.
(c)
(i) Seed ← Ovule
(ii) Fruit ← Ovary
OR
(a) Two reasons :
► Avoids unwanted/undesirable pregancies/ STD’s
► Use of condom prevents the transmission of infections from one person to another.
(b) Oral contraceptives change the hormonal balance of the body so that the eggs are not released.
(c) Sex selective abortion is a procedure that is done for female foetuses / female foeticide. It adversely affects the male-female sex ratio.
Question 29:
(a) For the combination of resistors shown in the following figure, find the equivalent resistance between M & N.
(b) State Joule’s law of heating.
(c) Why we need a 5 A fuse for an electric iron which consumes 1 kW power at 220 V ?
(d) Why is it impracticable to connect an electric bulb and an electric heater in series ?
Answer :
Question 30:
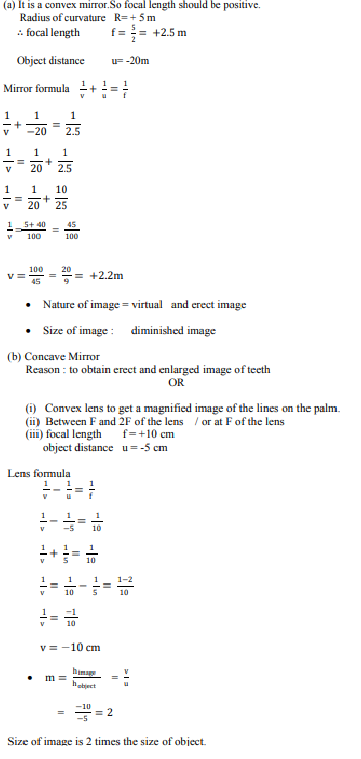
(a) A security mirror used in a big showroom has radius of curvature 5 m. If a customer is standing at a distance of 20 m from the cash counter, find the position, nature and size of the image formed in the security mirror.
(b) Neha visited a dentist in his clinic. She observed that the dentist was holding an instrument fitted with a mirror. State the nature of this mirror and reason for its use in the instrument used by dentist.
OR
Rishi went to a palmist to show his palm. The palmist used a special lens for this purpose.
(i) State the nature of the lens and reason for its use.
(ii) Where should the palmist place/hold the lens so as to have a real and magnified image of an object ?
(iii) If the focal length of this lens is 10 cm and the lens is held at a distance of 5 cm from the palm, use lens formula to find the position and size of the image.
Answer :
class 10 Science sample paper 2020 solved, sample paper class 10 Science, cbse class 10 Science question paper 2018, sample paper class 10 Science 2019, Science question paper for class 10, cbse previous year question papers class 10 Science, cbse sample paper for class 10 Science, cbse class 10 Science question paper 2019, class 10 Science sample paper 2019 solved, cbse class 10th Science question paper, 10th Science question paper, sample paper class 10 Science 2020, Science sample paper class 12, cbse question paper for class 9 Science, cbse Science sample paper, cbse 10th class Science
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.