
Here we are providing CBSE Previous Year Question Papers Class 6 to 12 solved with soutions Sample Paper Class 10 Science 2018 2019 2020 Standard CBSE Board U Like Arhint class 10 Science sample paper 2020 solved, sample paper class 10 Science, cbse class 10 Science question paper 2018, sample paper class 10 Science 2019, Science question paper for class 10, cbse previous year question papers class 10 Science, cbse sa Practice of previous year question papers and sample papers protects each and every student to score bad marks in exams.If any student of CBSE Board continuously practices last year question paper student will easily score high marks in tests. Fortunately earlier year question papers can assist the understudies with scoring great in the tests. Unraveling previous year question paper class 10 Science is significant for understudies who will show up for Class 10 Board tests.
Question 1: (Marks 1)
Covalent compounds are generally poor conductors of electricity. Why ?
Answer :
No charged particles/ions
Question 2: (Marks 1)
State the common characteristic of the following elements :
Boron, Silicon, Germanium and Arsenic
OR
State the Periodic Law on which the Modern Periodic Table is based.
Answer :
All are metalloids/Shows the properties of metals and non-metals
OR
Properties of elements are a periodic function of their atomic number
Question 3: (Marks 4)
Solar power in India is a fast developing industry. The country’s solar installed capacity reached 30·071 GW as of 31 July, 2019. India has the lowest capital cost per MW to install solar power plants. Solar electricity generation recorded nearly 3·4% of total utility electricity generation in January 2019. The following table shows Annual Solar Power Generation of the last six years.
Year | Solar Power Generation (TWh) |
2013 – 14 | 3·35 |
2014 – 15 | 4·60 |
2015 – 16 | 7·45 |
2016 – 17 | 12·09 |
2017 – 18 | 25·87 |
2018 – 19 | 39·27 |
Our country is lucky to receive solar energy for the greater part of the year. It is estimated that during a year India receives the energy equivalent to more than 5000 trillion kWh from the Sun.
3(a) What are solar cells ? (Marks 1)
3(b) How much voltage can be developed and how much electricity can be produced by one typical solar cell when exposed to the Sun ? (Marks 1)
3(c) The future of power generation by solar energy is bright in India. Give reason. (Marks 1)
3(d) List two advantages of solar cells. (Marks 1)
Answer :
(a) Cells which convert solar energy to electrical energy/electricity
(b) Voltage – 0.5 to 1V
Electricity –0.7W
(c) India receives great amount of solar energy throughout the year.
(d) Advantages :- No moving parts/require little maintenance /work quite satisfactorily without any focusing device/can be set up in remote and inaccessible areas. (Any Two)
Question 4: (Marks 4)
Question numbers 4(a) – 4(d) are based on the table and related information in the passage given below.
Thyroid Stimulating Hormone (TSH) stimulates thyroid gland to produce thyroxine. Study the table given below.
Stage of pregnancy | Normal (mU/L) | Low (mU/L) | High (mU/L) |
First trimester | 0·2 – 2·5 | < 0·2 | 2·5 – 10 |
Second trimester | 0·3 – 3·0 | < 0·3 | 3·01 – 4·5 |
Third trimester | 0·8 – 5·2 | < 0·8 | > 5·3 |
It is important to monitor TSH levels during pregnancy. High TSH levels and hypothyroidism can especially affect chances of miscarriage. Therefore, proper medication in consultation with a doctor is required to regulate/control the proper functioning of the thyroid gland.
4(a) Give the full form of TSH. (Marks 1)
4(b) State the main function of TSH. (Marks 1)
4(c) Why do TSH levels in pregnant women need to be monitored ? (Marks 1)
4(d) A pregnant woman has TSH level of 8·95 mU/L. What care is needed for her ? (Marks 1)
Answer :
(a) Thyroid stimulating hormone.
(b) It stimulates / regulates thyroid gland to produce thryroid hormone or thyroxine.
(c) Because high and low TSH level may increase the chances of miscarriage.
(d) Proper medication is required
Question 5: (Marks 1)
The image distance from the eye lens in the normal eye when we increase the distance of an object from the eye
(A) increases.
(B) decreases.
(C) remains unchanged.
(D) depends on the size of the eyeball
Answer :
(C) / remains unchanged
Question 6: (Marks 1)
The values of mA and μA are 1
(A) 10–6 A and 10–9 A respectively
(B) 10–3 A and 10–6 A respectively
(C) 10–3 A and 10–9 A respectively
(D) 10–6 A and 10–3 A respectively
Answer :
(B) / 10−3 A and 10−6 A respectively
Question 7: (Marks 1)
Answer :
(A) / 5A
Question 8: (Marks 1)
Consider the following criticisms that are generally addressed when a new project is launched :
I. Displacement of peasants and local tribals without compensation.
II. Swallowing up large amount of public money without any benefits.
III. Deforestation and loss of biodiversity.
The criticisms about large dams in particular are
(A) I and II
(B) II and III
(C) I and III
(D) I, II and III
OR
Switching off unnecessary lights and fans and repairing leaking taps correctly defines which term of 5R’s ?
(A) Recycle
(B) Reuse
(C) Repurpose
(D) Reduce
Answer :
(D) /I , II and III
OR
(D) / Reduce
Question 9: (Marks 1)
The Reni village of Garhwal is famous for
(A) Monocultures of pine, teak and eucalyptus.
(B) Chipko Movement.
(C) Extensive biodiversity.
(D) Participation of local people in efficient management of forests.
Answer :
(B)/ Chipko Movement
Question 10: (Marks 1)
Strong heating of ferrous sulphate leads to the formation of a brown solid and two gases. This reaction can be categorised as
(A) displacement and redox.
(B) decomposition and redox.
(C) displacement and endothermic.
(D) decomposition and exothermic.
Answer :
(B) / Decomposition & Redox
Question 11: (Marks 1)
If 10 mL of H2SO4 is mixed with 10 mL of Mg(OH)2 of the same concentration, the resultant solution will give the following colour with universal indicator :
(A) Red
(B) Yellow
(C) Green
(D) Blue
Answer :
(C)/ Green
Question 12: (Marks 1)
An element X with atomic number 12 forms a compound with element Y with atomic number 17. The formula of the compound formed is
(A) XY
(B) XY2
(C) X2Y
(D) X2Y3
OR
An element X is forming acidic oxide. Its most probable position in the modern periodic table is
(A) Group 1 and Period 3
(B) Group 16 and Period 3
(C) Group 17 and Period 3
(D) Group 2 and Period 3
Answer :
(B) / XY2
OR
(B ) / (C)
Group 16 and period 3 /Group 17 and period 3
(Note- Both are correct, marks to be awarded for any one)
(i) Both (A) and (R) are true and (R) is correct explanation of the assertion (A).
(ii) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion (A).
(iii) (A) is true, but (R) is false.
(iv) (A) is false, but (R) is true.
Question 13: (Marks 1)
Assertion (A) : Carbon has a strong tendency to either lose or gain electrons to attain noble gas configuration.
Reason (R) : Carbon has four electrons in its outermost shell and has the tendency to share electrons with carbon or other elements.
Answer :
(iv) / (A) is false, but (R) is true
Question 14: (Marks 1)
Assertion (A) : At high temperatures, metal wires have a greater chance of short circuiting.
Reason (R) : Both resistance and resistivity of a material vary with temperature.
Answer :
(ii) / Both (A) and (R) are true, but (R) is not the correct explanation of the assertion(A)
Question 15: (marks 3)
A shining metal ‘M’, on burning gives a dazzling white flame and changes to a white powder ‘N’.
(a) Identify ‘M’ and ‘N’.
(b) Represent the above reaction in the form of a balanced chemical equation.
(c) Does ‘M’ undergo oxidation or reduction in this reaction ? Justify.
Answer :
(a) ‘M’ is magnesium /Mg
‘N’ is Magnesium oxide / MgO
(b) 2Mg + O2 → 2MgO
(c) ‘M’ undergoes oxidation because oxygen is added to it/ Loss of 2 electrons
Question 16: (Marks 3)
In the electrolysis of water
(a) Name the gases liberated at anode and cathode.
(b) Why is it that the volume of gas collected on one electrode is two times that on the other electrode ?
(c) What would happen if dil. H2SO4 is not added to water ? 3
OR
A chemical compound ‘X’ is used in the soap and glass industry. It is prepared from brine.
(a) Write the chemical name, common name and chemical formula of ‘X’.
(b) Write the equation involved in its preparation.
(c) What happens when it is treated with water containing Ca or Mg salts ?
Answer :
Question 17: (Marks 3)
From the elements Li, K, Mg, C, Al, S identify the
(a) elements belonging to the same group.
(b) element which has the tendency to lose two electrons.
(c) element which prefers sharing of electrons to complete its octet.
(d) most metallic element.
(e) element that forms acidic oxide.
(f) element that belongs to group 13.
Answer :
(a) Li ,K
(b) Mg
(c) C
(d) K
(e) S
(f) Al
Question 18: (Marks 3)
What is meant by trophic level in a food chain ? Construct a terrestrial food chain with four trophic levels. The energy flow in a food chain is always unidirectional. Why ?
OR
Complete the following flow chart based on ecosystem and its components
Answer :
► Trophic level - Each step or level of a food chain forms a trophic level
► Grass → Insect → Frog →Snake/Hawk / Correct Diagram (any other)
► Because it moves progressively through the various trophic levels and is no longer available to the previous level from producers to consumers.
OR
(i) Aquatic
(ii) Abiotic
(iii) Air/Water/Soil/Temperature /Non-living
(iv) Living organism/plants and animals
(v) Definition – All the interacting organisms in an area together with the non living constituents of the environment form an ecosystem /interaction between biotic and abiotic components.
Question 19: (Marks 3)
Answer :
(a) Exchange of gases.
(b) Because amount of oxygen dissolved in water is fairly low as compared to the air
(c) ) (i) Pyruvate
(ii) Carbon dioxide
Question 20: (Marks 3)
(a) Why is the F1 progeny always of tall plants when a tall pea plant is crossed with a short pea plant ?
(b) How is F2 progeny obtained by self-pollination of F1 progeny different from F1 progeny ? Give reason for this observation.
(c) State a conclusion that can be drawn on the basis of this observation.
Answer :
(a) Because Tallness is the dominant trait
(b) The recessive character is expressed in the F2 generation when two copies of the recessive trait are present together/(tt).
(c) In the F2 progeny , the dominant character is also expressed along with the recessive character in ratio of 3:1 respectively
Question 21: (Marks 3)
(a) What provides nutrition to human sperms ? State the genetic constitution of a sperm.
(b) Mention the chromosome pair present in zygote which determines the sex of (i) a female child, and (ii) a male child.
Answer :
(a) ► Secretions from seminal vesicle.
► 22+X and 22+Y
(b) (i) Female-XX
(ii) Male – XY
Question 22: (Marks 3)
Draw ray diagram in each of the following cases to show what happens after reflection to the incident ray when
(a) it is parallel to the principal axis and falling on a convex mirror.
(b) it is falling on a concave mirror while passing through its principal focus.
(c) it is coming oblique to the principal axis and falling on the pole of a convex mirror.
Answer :
(Note : Deduct ½ marks overall if no arrows are shown)
Question 23: (Marks 3)
(a) A coil of insulated wire is connected to a galvanometer. What would be observed if a strong bar magnet with its south pole towards one face of the coil is
(i) moved quickly toward it ?
(ii) moved quickly away from it ?
(iii) held stationary near it ?
(b) Name the phenomena involved.
(c) State the conclusion based on the observations in (i), (ii) and (iii).
Answer :
(a) (i) Momentary deflection in the needle of the galvanometer to the left / right.
(ii) Momentary deflection in the needle of the galvanometer but in the opposite direction.
(iii) No deflection
(b) Electromagnetic induction.
(c) Motion of a magnet with respect to coil induces an electric current in the coil which lasts so long as the motion is taking place / change in magnetic field around a coil produces an induced current in it.
Question 24: (Marks 3)
A student uses spectacles of focal length – 2·5 m.
(a) Name the defect of vision he is suffering from.
(b) Which lens is used for the correction of this defect ?
(c) List two main causes of developing this defect. (d) Compute the power of this lens. 3
OR
Give reasons :
(a) Red colour is selected for danger signals.
(b) The sky appears dark in space.
(c) The time difference between actual sunset and apparent sunset is about 2 minutes.
Answer :
(a) Myopia/Short sightedness
(b) Concave/Diverging lens
Question 25: (Marks 5)
Two ores X and Y were taken. On heating these ores it was observed that
(a) ore X gives CO2 gas, and
(b) ore Y gives SO2 gas. Write steps to convert these ores into metals, giving chemical equations of the reactions that take place. 5
OR
(a) With the help of a diagram explain the method of refining of copper by electrolysis.
(b) How are broken railway tracks joined ? Give the name of the process and the chemical equation of the reaction involved.
Answer :
Fe2O3 with aluminum powder.
► Thermit process/reaction
► Fe2O3(s)+2Al(s) → 2Fe(l)+Al2O3(s)+Heat
Question 26: (Marks 5)
(a) Define isomerism. Draw all possible isomers of butane.
(b) ‘‘A compound ‘X’ on combustion gives a yellow flame with lots of smoke.’’ What inference would you draw from this statement ?
(c) State the role of alkaline KMnO4 in the reaction involving conversion of an alcohol to corresponding carboxylic acid.
Answer :
Question 27: (Marks 5)
Give reasons :
(a) Ventricles have thicker muscular walls than atria.
(b) Transport system in plants is slow.
(c) Circulation of blood in aquatic vertebrates differs from that in terrestrial vertebrates.
(d) During the daytime, water and minerals travel faster through xylem as compared to the night.
(e) Veins have valves whereas arteries do not.
Answer :
(a) Because ventricles have to pump blood to various distant organs of the body
(b) Because their energy requirement is low
(c) In aquatic vertebrates the blood goes only once through the heart during one cycle while in terrerstrial vertebrates it goes through the heart two times during each cycle.
(d) Because transpirational pull is greater during day time.
(e) To prevent the backflow of the blood /blood flows only in one direction
Question 28: (Marks 5)
Based on the given diagram answer the questions given below :
(a) Label the parts A, B, C and D.
(b) Name the hormone secreted by testis and mention its role.
(c) State the functions of B and C in the process of reproduction.
OR
(a) Name the mode of reproduction of the following organisms and state the important feature of each mode :
(i) Planaria
(ii) Hydra
(iii) Rhizopus
(b) We can develop new plants from the leaves of Bryophyllum. Comment.
(c) List two advantages of vegetative propagation over other modes of reproduction.
Answer :
(a)
► A→Ureter
► B→ Seminal Vesicle
► C→Urethra
► D→ Vas deferens
(b) Testosterone :
Role
► Regulates the formation of sperms
► Changes in appearance of boys at the time of puberty.
(c) Function of ‘B’
► Providing nutrition and transportation to sperms. Function of ‘C’
► Serves as a common passage to both sperms and urine.
OR
(a) ► Regeneration- the lost body part can be regenerated.
► Budding – a complete small individual develops on the parent body during favourable conditions.
► Spore Formation – Spores are covered with thick wall that helps to overcome unfavourable conditions.
(b) Buds produced in the notches along the leaf margins develop into new plants.
(c) Advantages :
► Propagation of flowerless plants.
► Genetically similar to the parent plant.
► Plants raised by vegetative propagation bear flowers and fruits earlier than those produced from seeds. (Any two)
Question 29: (Marks 5)
(a) Two lamps rated 100 W, 220 V and 10 W, 220 V are connected in parallel to 220 V supply. Calculate the total current through the circuit.
(b) Two resistors X and Y of resistances 2 Ω and 3 Ω respectively are first joined in parallel and then in series. In each case the voltage supplied is 5 V.
(i) Draw circuit diagrams to show the combination of resistors in each case.
(ii) Calculate the voltage across the 3 Ω resistor in the series combination of resistors.
Answer :
Question 30: (Marks 5)
(a) Draw a labelled ray diagram to show the path of a ray of light incident obliquely on one face of a glass slab.
(b) Calculate the refractive index of the material of a glass slab. Given that the speed of light through the glass slab is 2 × 108 m/s and in air is 3 × 108 m/s.
(c) Calculate the focal length of a lens, if its power is – 2·5 D. 5
OR
(a) A person suffering from myopia (near-sightedness) was advised to wear corrective lens of power 2·5 D. A spherical lens of same focal length was taken in the laboratory. At what distance should a student place an object from this lens so that it forms an image at a distance of 10 cm from the lens ?
(b) Draw a ray diagram to show the position and nature of the image formed in the above case.
Answer :
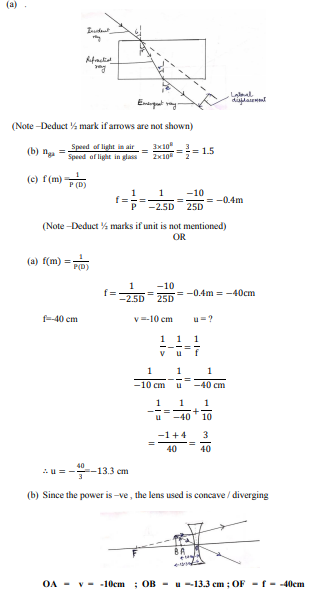
class 10 Science sample paper 2020 solved, sample paper class 10 Science, cbse class 10 Science question paper 2018, sample paper class 10 Science 2019, Science question paper for class 10, cbse previous year question papers class 10 Science, cbse sample paper for class 10 Science, cbse class 10 Science question paper 2019, class 10 Science sample paper 2019 solved, cbse class 10th Science question paper, 10th Science question paper, sample paper class 10 Science 2020, Science sample paper class 12, cbse question paper for class 9 Science, cbse Science sample paper, cbse 10th class Science
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.