
Here we are providing CBSE Previous Year Question Papers Class 6 to 12 solved with soutions CBSE Class 10 Science Question Paper 2017 2018 2019 2020 Solved all Sets class 10 Science sample paper 2020 solved, sample paper class 10 Science, cbse class 10 Science question paper 2018, sample paper class 10 Science 2019, Science question paper for class 10, cbse previous year question papers class 10 Science, cbse sa Practice of previous year question papers and sample papers protects each and every student to score bad marks in exams.If any student of CBSE Board continuously practices last year question paper student will easily score high marks in tests. Fortunately earlier year question papers can assist the understudies with scoring great in the tests. Unraveling previous year question paper class 10 Science is significant for understudies who will show up for Class 10 Board tests.
SECTION A
Question 1:
Name a cyclic unsaturated carbon compound.
Answer :
Cyclopentene / Cyclohexene-formula or structure (or any other). If candidate writes Benzene give full markQuestion 2:
The change in magnetic field lines in a coil is the cause of induced electric current in it. Name the underlying phenomenon.
Answer :
Electromagnetic InductionAnswer question numbers 3(a) to 3(d) and 4(a) to 4(d) on the basis of your understanding of the following paragraphs and the related studied concepts
Question 3:
The growing size of the human population is a cause of concern for all people. The rate of birth and death in a given population will determine its size. Reproduction is the process by which organisms increase their peopulation. The process of sexual maturation for reproduction is gradual and take place while general body growth is still going on. Some degree of sexual maturation does not necessarily mean that the mind or body is ready for sexual acts or for having and bringing up children. Various contraceptive devices are being used by human beings to control the size of population.
(a) List two common signs of sexual maturation in boys and girls.
(b)What is the result of reckless female foeticide?
(c) Which contraceptive method changes the hormonal balance of the body?
(d) Write two factors that determine the size of a population.
Answer:
a) Thick hair growth in armpits, genital area/thinner hair on arms, legs, face/ more active oil secretion from glands on skin/Occurrence of pimples (any two)
b) Imbalance in male – female ratio/ decline in child sex ratio
c) Oral pills
d) Rate of birth and death
Question 4:
Human body is made of five important components, of which water the main component. Food as well as potable water are essential for every human being. The food is obtained from plants through agriculture. Pesticides are being used extensively for a high yield in the fields. These pesticides are absorbed by the plants from soil along with water and minerals and from the water bodies these pesticides are taken up by the aquatic animals and plants. As these chemicals are not biodegradable, they get accumulated progressively at each trophic level. The maximum concentration of these chemicals gets accumulated in our bodies and greatly affects the health of our mind and body.
(a) Why is the maximum concentration of pesticides found in human beings?
(b) Give one method which could be applied to reduce our intake of pesticides through food to some extent.
(c) Various steps in a food chain represents:
| (a) Food web | (b) Trophic level |
| (c) Ecosystem | (d) Bimagnification |
(d) With regard to various food chains operating in an ecosystem, amn is a :
| (a) Consumer | (b) Producer |
| (c) Producer and consumer | (d) Producer and decomposer |
Answer :
| a) Human beings are at the top level in any food chain |
| b) Washing of vegetables, fruits, grains thoroughly/Organic farming/ Use of bio pesticides (any one) |
| c) (b) / Trophic level |
| d) (a) / Consumer |
Question 5:
Calcium oxide reacts vigorously with water to produce slaked lime.
CaO(s) + H2 O(l) → Ca(OH)2 (aq)
This reaction can be classified as :
(A) Combination reaction (B) Exothermic reaction
(C) Endothermic reaction (D) Oxidation reaction
Which of the following is a correct option?
(a) (A) and (C) (b) (C) and (D)
(c) (A), (c) and (D) (d) (A) and (B)
OR
When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of a :
(a) Combination raction (b) Displacement reaction
(c) Decomposition reaction (d) Double displacement reaction
Answer :
(d) / (A) and (B)
OR
(d)/ Double displacement reaction
Question 6:
In a double displscement reaction such as the reaction between sodium sulphate solution and barium chloride solution :
(A) exchange of atoms takes place (B) exchange ions takes places
(C) a precipitate is produced (D) an insoluble salt is produced
The correct option is :
(a) (B) and (D) (b) (A) and (C)
(c) only (B) (d) (B), (C) and (D)
Answer :
(d) / (B), (C) and (D)
Question 7:
Baking soda is a mixture of :
(a) Sodium carbonate and acetic acid
(b) Sodium carbonate and tartaric acid
(c) Sodium hydrogen carbonate and tataric acid
(d) Sodium hydrogen carbonate and acetic acid
Answer :
(c)/ Sodium hydrogen carbonate and tartaric acid
[Note: If a candidate writes ‘none of the options is correct’/ ‘sodium hydrogen carbonate’ give full credit.]
Question 8:
The chemical formula for plaster of Paris is :
Answer :
Question 9:
The laws of reflection hold true for :
| (a) plane mirrors onl;y | (b) concave mirrors onl;y |
| (c) Convex mirrors only | (d) all reflecting surfaces |
OR
When an object is kept within the focus of a concave mirror, an enlarged image is formed behind the mirror. This image is :
| (a) real | (b) inverted |
| (c) virtual and inverted | (d) virtual and erect |
Answer :
(d) / All reflecting surfaces
OR
(d) / Virtual and erect
Question 10:
At the time of short circuit, the electric current in the circuit :
| (a) Vary continuously | (b) does not change |
| (c) reduces substantially | (d) increases heavily |
OR
Two bulbs of 100 W and 40 W are connected in series. The current through the 100 W bulb is 1 A. The current through the 40 W bulb will be :
| (a) 0.4 A | (b) 0.6 A |
| (c) 0.8 A | (d) 1 A |
Answer :
(d) / Increases heavily
OR
(d) / 1A
Question 11:
Which one of the following is responsible for the sustenance of underground water?
(a) Loss of vegetation cover.
(b) Diversion for high water demanding crops.
(c) reduces global warming.
(d) Afforestation
Answer :
(d) / Afforestation
Question 12:
Incomplete combustion of coal and petroleum :
(A) increases air pollution.
(B) increases efficiency of machines.
(C) reduces global warming.
(D) produce poisonous gases.
The correct option is :
| (a) (A) and (B) | (b) (A) and (D) |
| (c) (B) and (C) | (d) (C) and (D) |
Answer :
(b) / (A) and (D)
For question numbers 13 and 14, two statements are given - one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below :
(a) Both A and R are true and R is correct explanation of the Assertion.
(b) Both A and R true but R is not the correct explanation of the Assertion.
(c) A is true but R is false
(d) A is false but R is true.
Question 13:
Assertion (A) : Esterification is a process in which a sweet smelling substance is produced.
Reason (R) : When esters react with sodium hydroxide an alcohol and sodium salt of carboxylic acid are obtained.
Answer :
(b) / Both (A) and (R) are true but (R) is not the correct explanation of the assertion (A).
Question 14:
Assertions (A) : In the process of nuclear fission, the amount of nuclear energy generated by the fission of an atom uranium is so treamendous that it produces 10 million times the nergy produced by the combustion of an atom of carbon from coal.
Rwason (R) : The nucleus of a heay atom such as uranium, when bornbarded with low energy neutrons, splits apart into lighter nuclei gets converted to tremendous energy.
Answer :
(a) / Both (A) and (R) are true and ( R) is the correct explanation of the assertion (A). 1 1 S
SECTION B
Question 15:
1 g of copper powder was taken in a China dish an heated. What change takes place on heating? When hydrogen gas is passed over this heated substance, a visible change is seen in it. Give the chemical equations of reactions, the name and the color of the products formed in each case.
Answer :
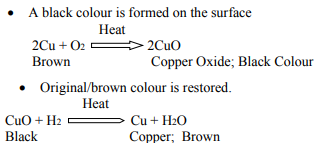
Question 16:
List the important products of the Clor-alkali process. Write one important use of each.
How is washing soda prepared from sodium carbonate? Give its chemical equation. State the type of this salt. Name the type of hardness of water which can be removed by it?
Answer :
Products: Hydrogen, Chlorine , Sodium hydroxide
Uses:
Hydrogen: In the production of margarine/ ammonia/as a fuel
Chlorine: Water treatment/ swimming pools/ production of PVC/ Disinfectants/CFCs/Pesticides.
Sodium hydroxide: For degreasing metal surfaces/ in making soaps and detergents/ paper making/ artificial fibres.
(any one use of these or any other)
OR
• By recrystallisation of sodium carbonate
• Na2CO3 + 10H2O → Na2CO3.10H2O
• Basic Salt
• Permanent hardness
Question 17:
3 mL of ethanol is taken in a test tube and warmed gently in a water bath. A 5% solution of alkaline potassium permanganate is added first drop by drop to this solution, then in excess.
(i) How is in 5% solution of KMnO4 prepared?
(ii) State the role of alkaline potassium permaganate in this reaction. What happens on adding it in excess
(iii) Write chemical equation of this reaction.
Answer :
Question 18:
A squirrel is in a scary situation. Its body has to prepare for either fighting or running away. State the immediate changes that take place in its body so that the squirrel is able to either fight or run?
OR
Why is chemical communication better than electrical impulses as a means of communication between cells in a multi-cellular organism?
Answer :
• The adrenaline hormone is secreted into the blood.
• The heart beats faster resulting in supply of more oxygen to the muscles.
• Blood is diverted to skeletal muscles.
• The breathing rate increases.
• The blood supply to digestive systems and skin is reduced.
OR
• Electrical impulses have limited access to only those cells that are connected by nervous tissue/ neurons, whereas chemical signals can reach each and every cell of the body .
• Cells need time to reset in order to create repeated/ new electrical impulses whereas no such time is required for chemical communication
Question 19:
Define the term pollination. Differentiate between self pollination and cross pollination. What is the significance of pollination?
Answer :
• Pollination is the transfer of pollen from anther to stigma
| Self Pollination | Cross Pollination |
| Transfer of pollen in the same flower | Transfer of pollen from one flower to another. |
• Pollination leads to fertilization resulting in the formation of zygote.
Question 20:
What are honologous structures? Give an example. Is it necessary that honologous structures always have a common ancestor. Justify your answer.
Answer :
• Homologous structures are those which have similar basic structure and origin but perform different functions.
• Example: forelimbs of reptiles, amphibians, humans, wings of birds
(or any other example)
• Yes
• Similarity in basic design of the structure indicates that their ancestors were common.
Question 21:
Why is Tyndall effect shown by colloidal particles? State four instances of observing the Tyndall effect.
OR
Differentiate between a glass slab and a glass prism. What happens when a narrow beam of (i) a monochromatic light, and (ii) white light passes through (a) glass slab and (b) glass prism?
Answer :
Because of scattering of light.
Instances:
• When a fine beam of light enters a smoke-filled dark room through a small hole.
• When sunlight passes through a canopy of dense forest in foggy/ misty conditions.
• Blue colour of sky.
• Red colour of the sun during sunrise or sunset.
(or any other)
OR
• Prism has 2 inclined refracting surfaces whereas a glass slab has 2 parallel refracting surfaces.
i) When monochromatic light passes through a glass slab it gets displaced laterally whereas in prism it gets angularly displaced.
ii) When white light passes through a glass slab, it gets laterally displaced whereas in prism, dispersion takes place.
Question 22:
Draw a labelled diagram to show (i) reddish appearance of the sun at the sunrise or the sunset and (ii) white appearance of the sun at noon when it is overhead.
Answer :
Question 23:
A V-I graph for a nichrome wire is given below. What do you infer from this graph? Draw a labelled circuit diagram to obtain such a graph.
Answer :
Question 24:
(a) Write the mathematical expression for Joule’s law of heacting.
(b) Compute the heat generated while transferring 96000 coulomb of charge in two hours through a potential difference of 40 V.
Answer :
i) H = I2Rt
ii) H = V.I.t
= V.Q
Given : V = 40 volts , Q = 96000 C
H = 40 V × 96000 C
= 3.84 × 106 J
SECTION C
Question 25:
Carbon cannot reduce the oxides of solution, magnesium and aluminium to their respective metals. Why? Where are these metals placed in the reactivity series? How are these metals obtained from their ores? Take an example to explain the process of extraction along with chemical equations.
Answer :
• These metals have more affinity for oxygen than carbon.
• Towards the top of the reactivity series .
• By electrotytic reduction of their molten ores.
• Example : Extraction of sodium from molten sodium chloride by electrolysis.
Process :
• Molten NaCl is taken in an electrolytic cell and on passing electricity Na is deposited at cathode and chlorine is liberated at anode .
Reactions –
At cathode - Na+ + e- → Na
At anode - 2Cl- → Cl2 + 2e-
( or any other example)
Question 26:
The position of certain elements in the Modern Periodic Table are shown below.
Using the above table answer the following questions giving reasons in each case :
(i) Which element will from only covalent compounds?
(ii) Which element is a non-metal with valency 2?
(iii) Which element is a metal with valency 2?
(iv) Out of H, C and F which has largest atomic size?
(v) To which family does H, C and F belong?
OR
Define atomic size. Given its unit of measurement. In the modem periodic table what trend is observed in the atomic radius in a group and a period and why is it so?
Answer :
i) E, it has 4 valence electrons .
ii) B, it needs only 2 electrons to attain stable configuration.
iii) D , it loses two electrons to attain stable configuration .
iv) F, it has the largest size since size increases down the group.
v) Noble gases, outermost shell is complete.
OR
• Atomic size is the distance between the centre of the nucleus and the outermost shell of an isolated atom.
• Picometer /pm
• Trends in Atomic radius
In a group: increases down the group ;
due to addition of a new shell .
In a period: atomic radius decreases from left to right ;
due to increase in pulling power of nucleus /
due to addition of electrons in the same shell.
Question 27:
(a) Why is there a difference in the rate of breathing between aquatic organisms and terrestrial organisms? Explain.
(b) Draw a diagram of human respiratory system and label - pharynx, trachea,lungs, diaphragm and alveolar sac on it.
(a) Name the organs that from the excretory system in human beings.
(b) Describe in brief how urine is produced in human body.
Answer :
a) Rate of breathing is faster in aquatic organisms because the amount of dissolved oxygen in water is lower as compared to the amount of oxygen in air.
b)
OR
b) A kidney has a large number of filteration units called nephrons. Each nephron has cup shaped Bowman’s capsule containing a bunch of capillaries called glomerulus. Blood gets filtered in the glomerulus. Filterate gets collected in Bowman’s capsule. Some useful substances such as glucose, amino acids, salts and water are selectively reabsorbed as urine flows through nephron tube. The urine formed in each kidney is eventually stored in the urinary bladder
Question 28:
(a) What is the law of dominance of traits? Explain with an example.
(b) Why are the traits acquired during the life time of an individual not inherited? Explain.
Answer :
a) Law of dominance of traits: -In a cross between a pair of contrasting characters, only one parental character will be expressed in F1 generation which is called dominant trait and the other is called recessive trait. For example – in pea plants,
All plants in F1 generation were tall proving that the gene for tallness is dominant over the gene for dwarfness/ short, which is not able to express itself in the presence of dominant trait.
(any other example)
b) Traits acquired by an organism during its lifetime are known as aquired traits.
These traits are not inherited because they occur in somatic cells only/do not cause any change in the DNA of the germ cells.
Question 29:
Draw a ray diagram in each of the following cases to show the formation of image, when the object is placed :
(i) between optical centre and principal focus of a convex lens.
(ii) anywhere in front of a concave lens.
(iii) at 2F of a convex lens.
State the signs and values of magnifications in the above mentioned cases (i) and (ii).
OR
An object 4.0 cm in size, is placed 25.0 cm in front of a concave mirror of focal length 15.0 cm.
(i) At what distance from the mirror should a screen be placed in order to obtain a sharp image?
(ii) Find the size of the image.
(iii) Draw a ray diagram to show the formation of image in this case.
Answer :
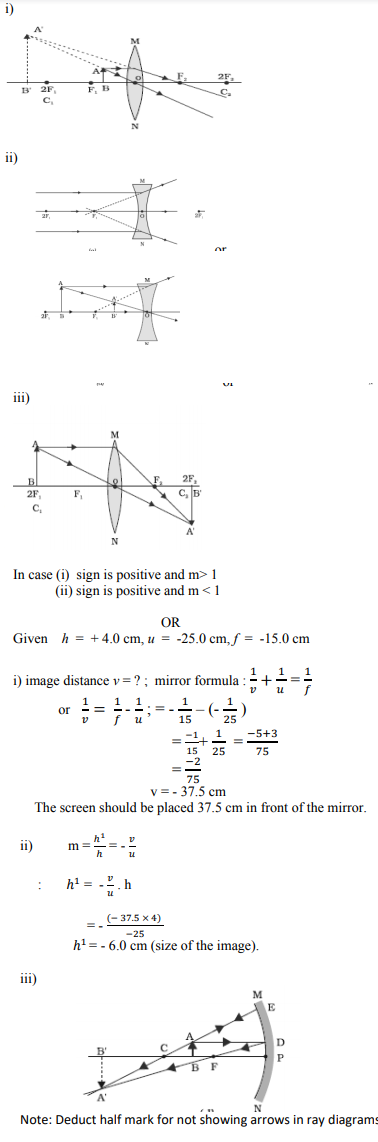
Question 30:
(a) What is an electromagnet ? List any two uses.
(b) Draw a labelled diagram to show how an electromagnet is made.
(c) State the purpose of soft iron core used in making an electromagnet.
(d) List two ways of increasing the strength of an electromagnet if the material of the electromagnet is fixed.
Answer :
a) A current carrying solenoid is called an electromageet /when soft iron is placed inside a solenoid carrying current, the soft iron piece behaves like a magnet so long as electric current passes through it. The magnet so formed is electromagnet.
Uses: In electric motors, electric bells, ( or any other )
(b)
(Direction of current)
c) Soft iron core is used to increase the strength/power of the electro magnet.
d) i) By increasing the current
ii) By increasing the number of turns in the coil.
class 10 Science sample paper 2020 solved, sample paper class 10 Science, cbse class 10 Science question paper 2018, sample paper class 10 Science 2019, Science question paper for class 10, cbse previous year question papers class 10 Science, cbse sample paper for class 10 Science, cbse class 10 Science question paper 2019, class 10 Science sample paper 2019 solved, cbse class 10th Science question paper, 10th Science question paper, sample paper class 10 Science 2020, Science sample paper class 12, cbse question paper for class 9 Science, cbse Science sample paper, cbse 10th class Science
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.