
Write the Nernst equation and emf of the following cells at 298 K Chapter 3: Electrochemistry Chemistry Class 12 solutions are developed for assisting understudies with working on their score and increase knowledge of the subjects. Question 5:Write the Nernst equation and emf of the following cells at 298 K: (i) Mg(s) | Mg2+(0.001M) || Cu2+(0.0001 M) | Cu(s) (ii) Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s) (iii) Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s) (iv) Pt(s) | Br2(l) | Br−(0.010 M) || H is solved by our expert teachers. You can get ncert solutions and notes for class 12 chapter 3 absolutely free. NCERT Solutions for class 12 Chemistry Chapter 3: Electrochemistry is very essencial for getting good marks in CBSE Board examinations
Question 5:Write the Nernst equation and emf of the following cells at 298 K:
(i) Mg(s) | Mg2+(0.001M) || Cu2+(0.0001 M) | Cu(s)
(ii) Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
(iii) Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
(iv) Pt(s) | Br2(l) | Br−(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).
Answer:
(i)Net reaction
Mg(s) +Cu2+(aq)↔ Mg2+(aq) + Cu(s)
Nernst equation
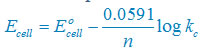
There are two electron are transferring so that n = 2
E°cell = E° right – E°left
E°cell =+0.34 – (–2.37)V
E°cell =+2.71V
Plug the value we get
Concentration of solid substance is 1 always so that [Mg] = [Cu] =1
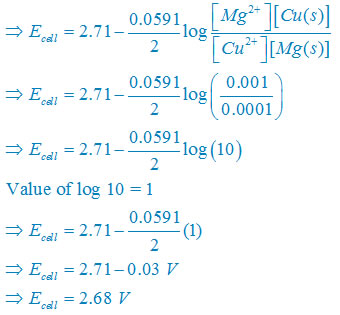
(ii) Net reaction
Fe(s) +2H+(aq)↔ Fe2+(aq) + H2(g)
Nernst equation
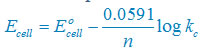
There are two electron are transferring so that n = 2
E°cell = E° right – E°left
E°cell =0– (–0.44)V
E°cell =+0.44 V
Plug the value we get
Concentration of solid substance is 1 always so that [Fe] = [H2] =1
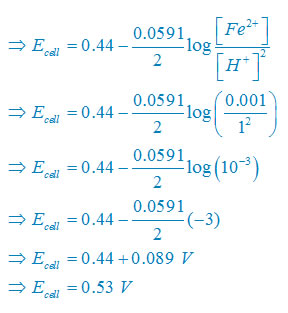
(iii) Net reaction
Sn(s) +2H+(aq)↔ Sn2+(aq) + H2(g)
Nernst equation
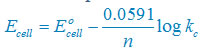
There are two electron are transferring so that n = 2
E°cell = E° right – E°left
E°cell =0– (–0.14)V
E°cell =+0.14 V
Plug the value we get
Concentration of solid substance is 1 always so that [Sn] = [H2] =1
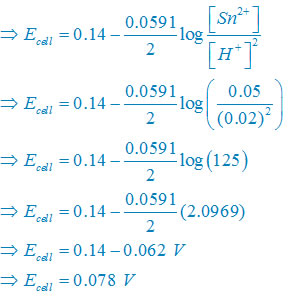
(iv) Net reaction
2Br–(aq) + 2H+(aq)↔ Br2(l) + H2(g)
Nernst equation
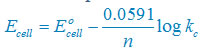
There are two electron are transferring so that n = 2
E°cell = E° right – E°left
E°cell =0– (1.08)V
E°cell –1.08V
Plug the value we get
Concentration of solid substance is 1 always so that [Br2] = [H2] =1
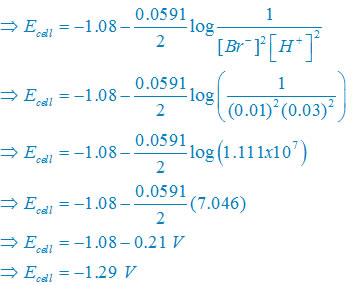
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.