
Question 4:Calculate the standard cell potentials of galvanic cells in which the following reactions take place: (i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd (ii) Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s) Calculate the =∆rGθ and equilibrium constant of the reactions. Chapter 3: Electrochemistry Chemistry Class 12 solutions are developed for assisting understudies with working on their score and increase knowledge of the subjects. Calculate the standard cell potentials of galvanic cells in which the following reactions take place: (i) 2Cr(s) + 3Cd2+(aq) ? 2Cr3+(aq) + 3Cd (ii) Fe2+(aq) + Ag+(aq) ? Fe3+(aq) + Ag(s) Calculate the =?rG? and equilibrium constant of the reactions. is solved by our expert teachers. You can get ncert solutions and notes for class 12 chapter 3 absolutely free. NCERT Solutions for class 12 Chemistry Chapter 3: Electrochemistry is very essencial for getting good marks in CBSE Board examinations
Question 4:Calculate the standard cell potentials of galvanic cells in which the following reactions
take place:
(i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd
(ii) Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s)
Calculate the =∆rGθ and equilibrium constant of the reactions.
Answer:(i)
The galvanic cell of the given reaction is represented as
Cr(s) | Cr3+ || Cd2+ | Cd(s)
The formula of standard cell potential is
E°cell = E° right – E°left
Click here to get all values
E°cell = E° Cd – R°Cr
E°cell = – 0.40 –( – 0.74 )
E°cell = – 0.40 –( – 0.74 )
E°cell = + 0.43 V
In balanced reaction there are 6 electron are transferring so that n = 6
Faraday constant, F = 96500 C mol−1
E°cell = + 0.34 V
Use formula
∆rGθ = – nFE°cell
Plug the value we get
Then, = −6 × 96500 C mol−1 × 0.34 V
= −196860 CV mol−1
= −196860J mol−1
= −196.86 kJ mol−1
Again,
Use second formula of ∆rGθ
∆rGθ = −2.303RT log kC
log KC = (∆rGθ) /( – 2.303RT)
plug the values we get
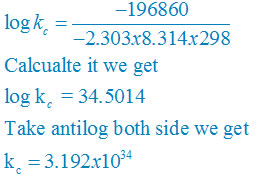
(ii)
The galvanic cell of the given reaction is represented as
Fe2+(aq) | Fe3+(aq) || Ag+ | Ag(s)
The formula of standard cell potential is
E°cell = E° right – E°left
Click here to get all values
E°cell = 0.80 – 0.77
E°cell = + 0.03 V
In balanced reaction there are 1 electron are transferring so that n = 1
Faraday constant, F = 96500 C mol−1
E°cell = + 0.03 V
Use formula
∆rGθ = – nFE°cell
Plug the value we get
Then, = −1 × 96500 C mol−1 × 0.03 V
= −2895 CV mol−1
= −2895J mol−1
= −2.895 kJ mol−1
Again,
Use second formula of ∆rGθ
∆rGθ = −2.303RT log kC
log KC = (∆rGθ) /( – 2.303RT)
plug the values we get
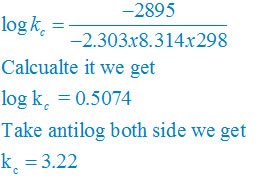
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.