
Question 7. How will you distinguish between the following pairs of terms (i) Hexagonal close packing and cubic close packing (ii) Crystal lattice and unit cell (iii) Tetrahedral void and octahedral void. Chapter 1: the Solid State Chemistry Class 12 solutions are developed for assisting understudies with working on their score and increase knowledge of the subjects. Hexagonal close packing and cubic close packing, Crystal lattice and unit cell, Tetrahedral void and octahedral void is solved by our expert teachers. You can get ncert solutions and notes for class 12 chapter 1 absolutely free. NCERT Solutions for class 12 Chemistry Chapter 1: the Solid State is very essencial for getting good marks in CBSE Board examinations
Question 7. How will you distinguish between the following pairs of terms
(i) Hexagonal close packing and cubic close packing
(ii) Crystal lattice and unit cell
(iii) Tetrahedral void and octahedral void.
Answer: (i)
Hexagonal close packing:–
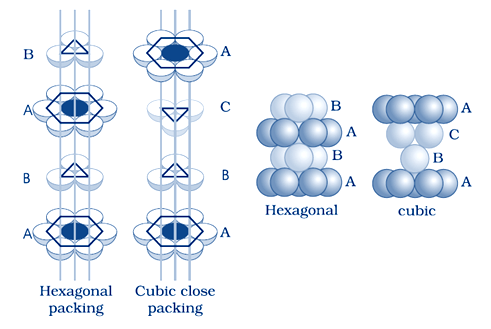
when the tetrahedral voids of the second layer is covered by the spheres of the third layer. so that the spheres of the third layer are exactly aligned with those of the first layer, we get a pattern of spheres which is repeated in alternate layers. This pattern can be written in the form of ABAB ....... pattern. This structure is called hexagonal close packed (hcp) structure. Magnesium and zinc metals have this pattern
cubic close packing:– When the third layer is placed above the second layer in a manner such that its spheres cover the octahedral voids. and the spheres of the third layer are not aligned with first or the second layer, arrangement is called “C’ type.This pattern of layers is often written as ABCABC ........... This structure is called cubic close packed (ccp)
(ii)Crystal lattice :–
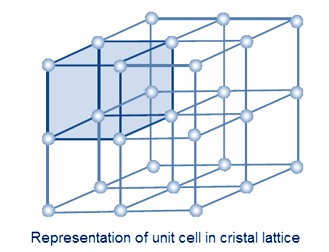
If the constituent particles are represented by dot (.) ,The periodic and systematic arrangement of these dots in three dimensions is called Crystal lattice.
Or
If each particle in three dimensional arrangement of constituent particles in a crystal is represented by a point, the arrangement is called crystal lattice. Thus, a regular three dimensional arrangement of constituent particles which represented by points in space is called a crystal lattice.
Unit cell :– the smallest repeating unit in space lattice is called unit cell. When we repeat unit cell in all dimensions, we get a structure of the crystal. Properties of unit cell are measured by length of edges and the angles between the edges.
(iii) Tetra hendral void :–
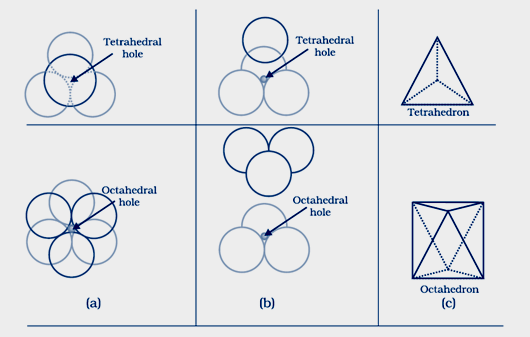
When a sphere of second layer is touching three sphere of first layer and the centers of all four sphere are at the corners of tetrahedron, the empty space which has tetra hendral arrangement is called tetra hedral void.
Octahedral void :–
When the tetrahedral void are formed at the corners of six sphere, each octa hedral void generate two set of equilateral triangles which are opposite to each other are called octahedral voids
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.