
Question 23: Explain the following terms with suitable examples: (i) Schottky defect (ii) Frenkel defect (iii) Interstitials and (iv) F–centres Chapter 1: the Solid State Chemistry Class 12 solutions are developed for assisting understudies with working on their score and increase knowledge of the subjects. Explain the following terms with suitable examples: (i) Schottky defect (ii) Frenkel defect (iii) Interstitials and (iv) F–centres is solved by our expert teachers. You can get ncert solutions and notes for class 12 chapter 1 absolutely free. NCERT Solutions for class 12 Chemistry Chapter 1: the Solid State is very essencial for getting good marks in CBSE Board examinations
Question 23: Explain the following terms with suitable examples:
(i) Schottky defect
(ii) Frenkel defect
(iii) Interstitials and
(iv) F–centres
Answer (i) Schottky Defect:
It is basically a vacancy defect in ionic solids. In order to maintain electrical neutrality, the number of missing cations and anions are equal. Like simple vacancy defect, Schottky defect also decreases the density of the substance. Number of such defects in ionic solids is quite significant. 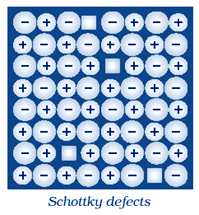
(ii) Frenkel Defect:
This defect is shown by ionic solids. In this defect the smaller ions are dislocated from its normal site to an interstitial site . It generate a vacancy defect at its original site and an interstitial defect at itsnew location. Frenkel defect is also called dislocation defect.
In this type of defect, density remains same. Frenkel defect is shown by ionic substance in which there is a large difference in the size of ions, for example, ZnS, AgCl, AgBr and AgI shows this effect due to small size of Zn2+ and Ag+ ions.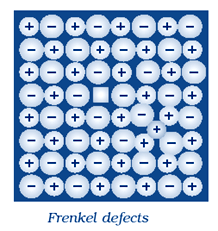
For example, in NaCl there are approximately 106 Schottky pairs per cm3 at room temperature. In 1 cm3 there are about 1022 ions. Thus, there is one Schottky defect per 1016 ions. Schottky defect is shown by ionic substances in which the cation and anion are of almost similar sizes. For example, NaCl, KCl, CsCl and AgBr. It may be noted that AgBr shows both, Frenkel as well as Schottky defects.
(iii) Interstitial Defect:
When some constituent particles(atoms or molecules) occupy an interstitial site,the crystal is said to have interstitial defect. This defect increases the density of the substance.Vacancy and interstitial defects as explained above can be shown by non–ionic solids. Ionic solids must always maintain electrical neutrality.Rather than simple vacancy or interstitial defects, they show these defects as Frenkel and Schottky defects.
(iv) F–centres:
A compound may have excess metal ion. When negative ion is absent from its lattice side and compound have excess metal ion and leaving a hole which occupied by electron to maintain electrical neutrality , the ionic sites are called F center .Colour in these crystals are impart by unpaired electrons
These types of defects are found in crystals which have electric neutrality and Schoottky defects .The hole occupied by electron is called F–center. This F center is responsible for most of the interstitial properties of compound
For example We observe this effect when Crystals of NaCl heated in an atmosphere of sodium vapour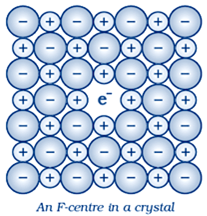
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.