
Explain sublimation. chapter 2 can study by students of class 9. These definitiona and formulas of Class 9 Science Chapter 2: is Matter Around Us Pure is developed and witten by our expert teachers. Science formulas. Explain sublimation. is prepapred and collected from varius resources to help the students.
Explain sublimation.
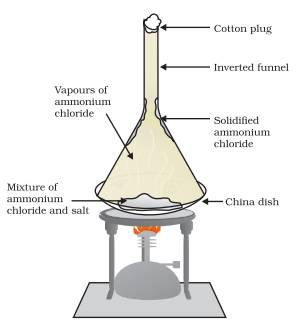
Answer: Sublimation is the property of substance in which they are converted directly from solid to gas or vice versa. Such substances are known as sublime. Some examples of solids which sublime are ammonium chloride, camphor, naphthalene and anthracene. Let us perform an activity to separate a mixture of ammonium chloride and salt.
Take a mixture of ammonium chloride and salt in a china dish cover it inverted conical transparent funnel. At the other end of the funnel put a cotton plug so that vapour could not come out. Now place china dish on a burner. As the ammonium chloride is sublime after heating it will directly converted into vapour and this vapour will again condense at the upper colder part of funnel to form solid ammonium chloride. In this way the mixture ammonium chloride and salt can be separated by the sublimation method.
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.